当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the Hydrogen Bond Interactions in Deep Eutectic Solvents Composed of Choline Chloride and Polyols
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-03-19 00:00:00 , DOI: 10.1021/acssuschemeng.8b06676 Huiyong Wang 1, 2 , Shuyan Liu 2 , Yuling Zhao 2 , Jianji Wang 2 , Zhiwu Yu 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-03-19 00:00:00 , DOI: 10.1021/acssuschemeng.8b06676 Huiyong Wang 1, 2 , Shuyan Liu 2 , Yuling Zhao 2 , Jianji Wang 2 , Zhiwu Yu 1
Affiliation
|
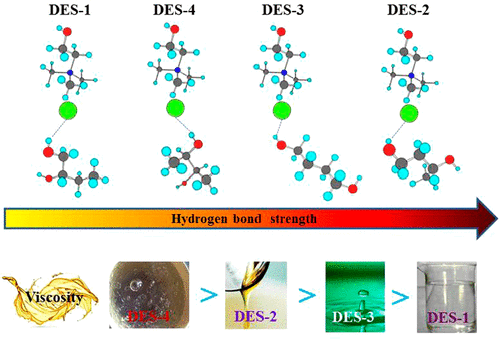
Deep eutectic solvents (DESs) consisting of cholinium chloride (ChCl) and alcohols have been widely applied in the purification of bioactive compounds, biodiesel, and flavonoids. However, an explicit and complete knowledge of the interactions between ChCl and alcohols is still lacking. In this work, the interactions between ChCl and polyols (1,2-butanediol, 1,3-butanediol, 1,4-butanediol, 2,3-butanediol, 1,3-propanediol, glycerol, 1,5-pentanediol, 1,2,5-pentanetriol, and xylitol) have been investigated at different molar ratios of ChCl to polyols by Fourier transform middle infrared, far-infrared, 1H and 35Cl NMR spectroscopy as well as quantum chemistry calculation. It is shown that hydrogen bond interaction between Cl atom of ChCl and H atom of the O-H group in the polyols is predominant and its strength decreases with both the increase of carbon number between two hydroxyl groups in butanediol and the decrease of hydroxyl number in polyols. As butanediol content is increased in the mixture, the hydrogen bond interaction of choline cations with Cl– is weakened, whereas that among butanediol molecules is enhanced. A molar ratio of ChCl to butanediol at 1:2 is indispensable for the formation of DES in a reasonable strength of hydrogen bond. The present results offer a possible explanation for viscosities of the DESs and may afford useful knowledge for the design and development of new DESs.
中文翻译:

对由氯化胆碱和多元醇组成的深共晶溶剂中氢键相互作用的见解
由氯化胆碱(ChCl)和醇组成的深共熔溶剂(DES)已广泛用于纯化生物活性化合物,生物柴油和类黄酮。然而,仍然缺乏对ChCl和醇之间相互作用的明确和完全的了解。在这项工作中,ChCl与多元醇(1,2-丁二醇,1,3-丁二醇,1,4-丁二醇,2,3-丁二醇,1,3-丙二醇,甘油,1,5-戊二醇,1 (1,2,5-戊三醇和木糖醇)已通过傅里叶变换中红外,远红外,1 H和35在不同的氯仿与多元醇的摩尔比下进行了研究Cl NMR光谱以及量子化学计算。结果表明,多元醇中ChCl的Cl原子与OH的H原子之间的氢键相互作用占主导地位,并且强度随着丁二醇中两个羟基之间碳原子数的增加和多元醇中羟基数的减少而降低。丁二醇含量在混合物中增加,有Cl胆碱阳离子的氢键相互作用-减弱,而该丁二醇分子之间被增强。ChCl与丁二醇的摩尔比为1:2对于以合理的氢键强度形成DES是必不可少的。本结果为DES的粘度提供了可能的解释,并可能为新DES的设计和开发提供有用的知识。
更新日期:2019-03-19
中文翻译:

对由氯化胆碱和多元醇组成的深共晶溶剂中氢键相互作用的见解
由氯化胆碱(ChCl)和醇组成的深共熔溶剂(DES)已广泛用于纯化生物活性化合物,生物柴油和类黄酮。然而,仍然缺乏对ChCl和醇之间相互作用的明确和完全的了解。在这项工作中,ChCl与多元醇(1,2-丁二醇,1,3-丁二醇,1,4-丁二醇,2,3-丁二醇,1,3-丙二醇,甘油,1,5-戊二醇,1 (1,2,5-戊三醇和木糖醇)已通过傅里叶变换中红外,远红外,1 H和35在不同的氯仿与多元醇的摩尔比下进行了研究Cl NMR光谱以及量子化学计算。结果表明,多元醇中ChCl的Cl原子与OH的H原子之间的氢键相互作用占主导地位,并且强度随着丁二醇中两个羟基之间碳原子数的增加和多元醇中羟基数的减少而降低。丁二醇含量在混合物中增加,有Cl胆碱阳离子的氢键相互作用-减弱,而该丁二醇分子之间被增强。ChCl与丁二醇的摩尔比为1:2对于以合理的氢键强度形成DES是必不可少的。本结果为DES的粘度提供了可能的解释,并可能为新DES的设计和开发提供有用的知识。































 京公网安备 11010802027423号
京公网安备 11010802027423号