Nature Catalysis ( IF 42.8 ) Pub Date : 2019-03-18 , DOI: 10.1038/s41929-019-0249-z Ombeline Mayol , Karine Bastard , Lilian Beloti , Amina Frese , Johan P. Turkenburg , Jean-Louis Petit , Aline Mariage , Adrien Debard , Virginie Pellouin , Alain Perret , Véronique de Berardinis , Anne Zaparucha , Gideon Grogan , Carine Vergne-Vaxelaire
|
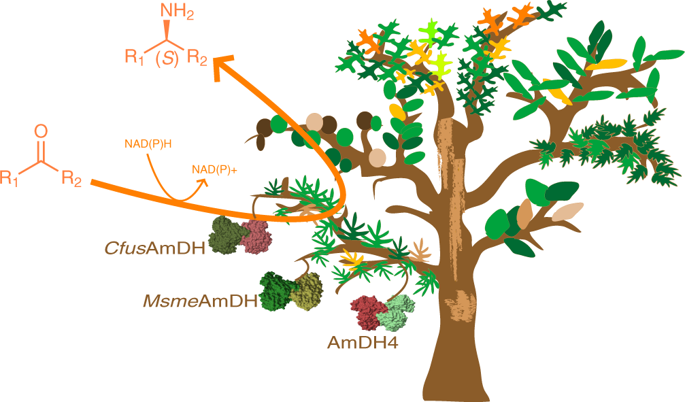
The asymmetric reductive amination of ketones enables the one-step synthesis of chiral amines from readily available starting materials. Here we report the discovery of a family of native NAD(P)H-dependent amine dehydrogenases (nat-AmDHs) competent for the asymmetric reductive amination of aliphatic and alicyclic ketones, adding significantly to the biocatalytic toolbox available for chiral amine synthesis. Studies of ketone and amine substrate specificity and kinetics reveal a strong preference for aliphatic ketones and aldehydes, with activities of up to 614.5 mU mg−1 for cyclohexanone with ammonia, and 851.3 mU mg−1 for isobutyraldehyde with methylamine as the amine donor. Crystal structures of three nat-AmDHs (AmDH4, MsmeAmDH and CfusAmDH) reveal the active site determinants of substrate and cofactor specificity and enable the rational engineering of AmDH4 for the generated activity towards pentan-2-one. Analysis of the three-dimensional catalytic site distribution among bacterial biodiversity revealed a superfamily of divergent proteins with representative specificities ranging from amino acid substrates to hydrophobic ketones.
中文翻译:

天然胺脱氢酶家族,用于酮的不对称还原胺化
酮的不对称还原胺化使得可以从容易获得的起始原料一步合成手性胺。在这里我们报告发现了一个天然的NAD(P)H依赖的胺脱氢酶(nat-AmDHs)家族的发现,该家族可用于脂肪族和脂环族酮的不对称还原胺化,大大增加了可用于手性胺合成的生物催化工具箱。酮和胺底物特异性和动力学的研究揭示了脂族酮和醛的强烈偏好,具有高达614.5 MU毫克的活动-1为环己酮与氨,和851.3毫克MU -1异丁醛与甲胺作为胺供体。三种nat-AmDHs(AmDH4,Msme AmDH和Cfus的晶体结构AmDH)揭示了底物和辅因子特异性的活性位点决定因素,并使AmDH4能够合理地工程化生成的对戊烷-2-一的活性。对细菌生物多样性中三维催化位点分布的分析揭示了具有差异性蛋白质的超家族,其代表性特异性范围从氨基酸底物到疏水性酮。

































 京公网安备 11010802027423号
京公网安备 11010802027423号