Nature Catalysis ( IF 42.8 ) Pub Date : 2019-03-18 , DOI: 10.1038/s41929-019-0250-6 Sobi Asako , Hirotaka Nakajima , Kazuhiko Takai
|
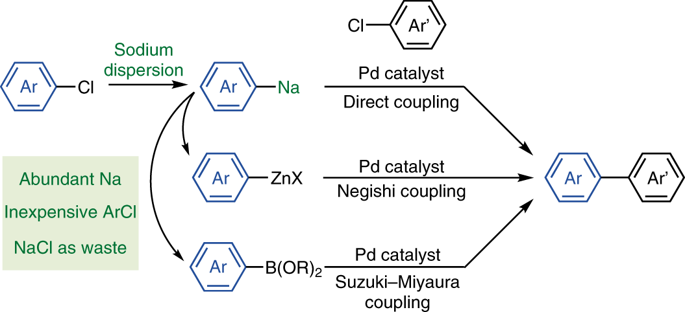
Sodium is the most abundant alkali metal in the Earth’s crust and the ocean. However, organosodium compounds have long been considered inferior to organolithium compounds, which have instead dominated synthetic organic chemistry during the last century. Despite being largely neglected because of their reactive nature, it is worth re-exploring organosodium chemistry, in light of the growing demand for sustainable syntheses without recourse to less abundant elements such as lithium. Herein, we demonstrate that, contrary to common belief, organosodium compounds can be easily prepared from aryl chlorides or (hetero)arenes and easy-to-handle sodium dispersion and, after being transmetallated to the corresponding zinc and boron compounds, they readily participate in the Negishi and Suzuki–Miyaura cross-coupling reactions, fundamental carbon–carbon bond-forming reactions in organic synthesis. Direct coupling reactions with organosodium species were also possible.
中文翻译:

用于催化交叉偶联的有机钠化合物
钠是地壳和海洋中最丰富的碱金属。但是,长期以来人们一直认为有机钠化合物不如有机锂化合物,后者在上个世纪主导了合成有机化学。尽管由于其反应性而在很大程度上被忽略,但鉴于对可持续合成的需求不断增长,而又不依赖锂等含量较低的元素,值得重新探索有机钠的化学性质。在此,我们证明,与通常的看法相反,有机钠化合物可以很容易地从芳基氯化物或(杂)芳烃和易于处理的钠分散体制备,并且在被金属化为相应的锌和硼化合物后,它们很容易地参与Negishi和Suzuki-Miyaura的交叉耦合反应,有机合成中基本的碳-碳键形成反应。与有机钠物质的直接偶联反应也是可能的。





























 京公网安备 11010802027423号
京公网安备 11010802027423号