当前位置:
X-MOL 学术
›
Nutr. Diabetes
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Responsiveness of hypothalamo-pituitary-adrenal axis to leptin is impaired in diet-induced obese rats.
Nutrition & Diabetes ( IF 4.6 ) Pub Date : 2019-03-18 , DOI: 10.1038/s41387-019-0076-y Andrew C Shin 1 , Sheba M J MohanKumar 2 , Priya Balasubramanian 3 , Madhu P Sirivelu 3 , Katrina Linning 3 , Andrew Woolcock 3 , Michelle James 3 , Puliyur S MohanKumar 2
Nutrition & Diabetes ( IF 4.6 ) Pub Date : 2019-03-18 , DOI: 10.1038/s41387-019-0076-y Andrew C Shin 1 , Sheba M J MohanKumar 2 , Priya Balasubramanian 3 , Madhu P Sirivelu 3 , Katrina Linning 3 , Andrew Woolcock 3 , Michelle James 3 , Puliyur S MohanKumar 2
Affiliation
|
BACKGROUND/OBJECTIVES
Diet-induced obese (DIO) rats have altered stress (HPA) axis activity compared to diet-resistant (DR) rats when chronically exposed to a high-fat (HF) diet. Since stress axis is tightly regulated by leptin, an adipocyte-secreted hormone that is important for controlling body weight, we hypothesized that leptin action is impaired in DIO rats leading to alterations in HPA axis activity.
SUBJECTS/METHODS
We intraperitoneally injected selectively bred DIO and DR rats with either saline or recombinant rat leptin. HPA axis activity was assessed by measuring norepinephrine (NE) in the paraventricular nucleus (PVN), corticotropin-releasing hormone (CRH) in the median eminence, and serum corticosterone (CORT). To test if HF exposure duration and the corresponding increase in leptin differentially affects HPA axis activity, we placed animals on a chow or HF diet for 1 or 6 weeks.
RESULTS
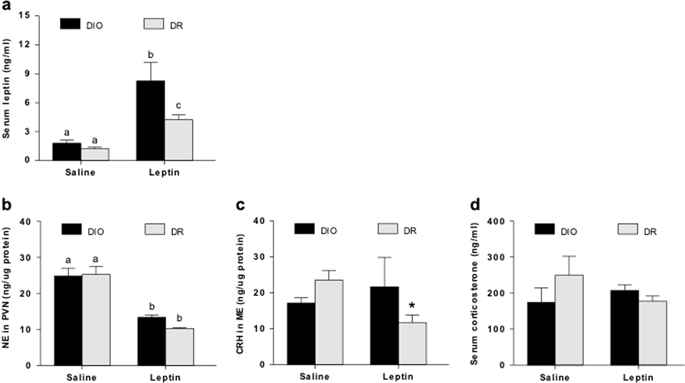
Leptin injection significantly increased serum leptin levels in both DIO and DR animals. It also reduced PVN NE in both groups, indicating that noradrenergic neurons in both groups remain responsive to leptin. HF diet duration-dependently increased serum leptin only in DIO animals whereas PVN NE increased in both groups. While DR rats responded to HF diet by increasing CRH and CORT at both time-points, responses in DIO rats varied, suggesting that they have altered HPA axis activity that may be dependent on HF-induced leptin levels and/or signaling. To understand the underlying mechanisms, we measured pSTAT-3, a marker of leptin signaling, in brainstem noradrenergic neurons and found reduced pSTAT-3 in A1 region of HF-fed DIO rats. We also found higher serum free fatty acids (FFAs) and a pro-inflammatory cytokine, IL-1β.
CONCLUSIONS
Collectively, these findings reveal that DIO rats have inherent neuroendocrine impairment in NE-HPA axis circuitry that worsens with the extent of HF diet exposure, possibly due to brainstem leptin resistance and/or elevated circulating FFAs and IL-1β.
中文翻译:

在饮食诱导的肥胖大鼠中,下丘脑-垂体-肾上腺轴对瘦素的反应性受损。
背景/目的与长期饮食习惯(高脂饮食)的饮食抵抗(DR)大鼠相比,饮食诱导肥胖(DIO)大鼠的应激(HPA)轴活性发生了改变。由于应激轴是由瘦素严格控制的,瘦素是一种对体重控制很重要的脂肪细胞分泌激素,因此我们假设瘦素的作用在DIO大鼠中受损,导致HPA轴活性改变。受试者/方法我们腹膜内注射盐水或重组大鼠瘦素选择性繁殖的DIO和DR大鼠。通过测量脑室旁核(PVN)中的去甲肾上腺素(NE),中位隆起中的促肾上腺皮质激素释放激素(CRH)和血清皮质酮(CORT)来评估HPA轴活性。为了测试HF暴露时间和瘦素的相应增加是否会对HPA轴活性产生差异影响,我们将动物置于食物或HF饮食中1或6周。结果瘦素注射液可显着增加DIO和DR动物的血清瘦素水平。它还降低了两组的PVN NE,表明两组中的去甲肾上腺素能神经元仍然对瘦素有反应。HF饮食持续时间仅在DIO动物中依赖性地增加血清瘦素,而PVN NE在两组中均增加。尽管DR大鼠在两个时间点都通过增加CRH和CORT对HF饮食做出了反应,但DIO大鼠的反应却有所不同,表明它们已经改变了HPA轴活性,这可能取决于HF诱导的瘦素水平和/或信号传导。为了了解潜在的机制,我们在脑干去甲肾上腺素能神经元中测量了瘦素信号转导的标志物pSTAT-3,发现在HF喂养的DIO大鼠的A1区域pSTAT-3减少。我们还发现了较高的血清游离脂肪酸(FFA)和促炎细胞因子IL-1β。结论总体而言,这些发现表明,DIO大鼠在NE-HPA轴电路中固有的神经内分泌功能障碍,随着HF饮食暴露程度的增加而恶化,这可能是由于脑干瘦素抵抗力和/或循环FFA和IL-1β升高所致。
更新日期:2019-03-18
中文翻译:

在饮食诱导的肥胖大鼠中,下丘脑-垂体-肾上腺轴对瘦素的反应性受损。
背景/目的与长期饮食习惯(高脂饮食)的饮食抵抗(DR)大鼠相比,饮食诱导肥胖(DIO)大鼠的应激(HPA)轴活性发生了改变。由于应激轴是由瘦素严格控制的,瘦素是一种对体重控制很重要的脂肪细胞分泌激素,因此我们假设瘦素的作用在DIO大鼠中受损,导致HPA轴活性改变。受试者/方法我们腹膜内注射盐水或重组大鼠瘦素选择性繁殖的DIO和DR大鼠。通过测量脑室旁核(PVN)中的去甲肾上腺素(NE),中位隆起中的促肾上腺皮质激素释放激素(CRH)和血清皮质酮(CORT)来评估HPA轴活性。为了测试HF暴露时间和瘦素的相应增加是否会对HPA轴活性产生差异影响,我们将动物置于食物或HF饮食中1或6周。结果瘦素注射液可显着增加DIO和DR动物的血清瘦素水平。它还降低了两组的PVN NE,表明两组中的去甲肾上腺素能神经元仍然对瘦素有反应。HF饮食持续时间仅在DIO动物中依赖性地增加血清瘦素,而PVN NE在两组中均增加。尽管DR大鼠在两个时间点都通过增加CRH和CORT对HF饮食做出了反应,但DIO大鼠的反应却有所不同,表明它们已经改变了HPA轴活性,这可能取决于HF诱导的瘦素水平和/或信号传导。为了了解潜在的机制,我们在脑干去甲肾上腺素能神经元中测量了瘦素信号转导的标志物pSTAT-3,发现在HF喂养的DIO大鼠的A1区域pSTAT-3减少。我们还发现了较高的血清游离脂肪酸(FFA)和促炎细胞因子IL-1β。结论总体而言,这些发现表明,DIO大鼠在NE-HPA轴电路中固有的神经内分泌功能障碍,随着HF饮食暴露程度的增加而恶化,这可能是由于脑干瘦素抵抗力和/或循环FFA和IL-1β升高所致。

































 京公网安备 11010802027423号
京公网安备 11010802027423号