Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2019-03-18 , DOI: 10.1016/j.apcatb.2019.03.047 Minghui Zhu , Jiacheng Chen , Rong Guo , Jing Xu , Xiangchen Fang , Yi-Fan Han
|
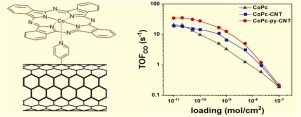
Electrochemical reduction of CO2 is promising to utilize the intermittent renewable electricity and transform CO2 into value-added products, simultaneously. Herein, we designed a cobalt phthalocyanine-based catalyst supported on pyridine-functionalized carbon nanotubes (CoPc-py-CNT). This novel hybrid catalyst exhibited a high activity (TOFCO: 34.5 s−1 at −0.63 V vs. RHE) and selectivity (FECO > 98%) for electrochemical CO2 reduction. To the best of our knowledge, it is the best one among all reported molecular based electrocatalysts for CO2-to-CO conversion. Furthermore, structure characterizations (such as Raman and X-rays photoelectron spectroscopy), loading-dependent electrochemical analysis and mechanistic studies revealed that pyridine groups, through axial coordination with Co, not only functioned as physical promoters to improve the dispersion of cobalt phthalocyanine but also tuned the electronic structure of Co sites to increase the intrinsic turnover frequency.
中文翻译:

酞菁钴与吡啶官能化的碳纳米管配位,并具有增强的CO 2电还原
电化学还原CO 2有望利用间歇性可再生电力并将CO 2同时转化为增值产品。在本文中,我们设计了负载在吡啶官能化碳纳米管(CoPc-py-CNT)上的酞菁钴基催化剂。这种新颖的杂化催化剂表现出高活性(TOF CO:-0.63 V下相对于RHE的TOF CO:34.5 s -1)和 电化学还原CO 2的选择性(FE CO > 98%)。据我们所知,它是所有报道的基于分子的CO 2电催化剂中最好的一种-CO转换。此外,结构表征(例如拉曼和X射线光电子能谱),依赖于负载的电化学分析和机理研究表明,吡啶基团通过与Co轴向配位,不仅起到物理促进剂的作用,以改善钴酞菁的分散性,而且调整了Co位点的电子结构,以增加内在周转频率。






























 京公网安备 11010802027423号
京公网安备 11010802027423号