当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrophobic Collapse in N-Methylacetamide-Water Mixtures.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-22 , DOI: 10.1021/acs.jpca.8b00276 Evgeniia Salamatova 1 , Ana V Cunha 1 , Robbert Bloem 2 , Steven J Roeters 2 , Sander Woutersen 2 , Thomas L C Jansen 1 , Maxim S Pshenichnikov 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-22 , DOI: 10.1021/acs.jpca.8b00276 Evgeniia Salamatova 1 , Ana V Cunha 1 , Robbert Bloem 2 , Steven J Roeters 2 , Sander Woutersen 2 , Thomas L C Jansen 1 , Maxim S Pshenichnikov 1
Affiliation
|
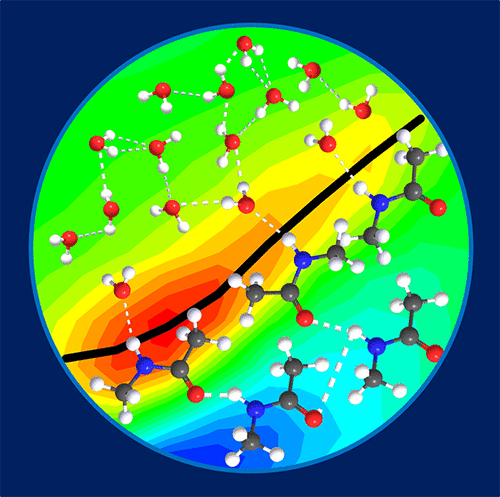
Aqueous N-methylacetamide solutions were investigated by polarization-resolved pump-probe and 2D infrared spectroscopy (2D IR), using the amide I mode as a reporter. The 2D IR results are compared with molecular dynamics simulations and spectral calculations to gain insight into the molecular structures in the mixture. N-Methylacetamide and water molecules tend to form clusters with "frozen" amide I dynamics. This is driven by a hydrophobic collapse as the methyl groups of the N-methylacetamide molecules cluster in the presence of water. Since the studied system can be considered as a simplified model for the backbone of proteins, the present study forms a convenient basis for understanding the structural and vibrational dynamics in proteins. It is particularly interesting to find out that a hydrophobic collapse as the one driving protein folding is observed in such a simple system.
中文翻译:

N-甲基乙酰胺-水混合物中的疏水塌陷。
使用酰胺 I 模式作为报告基因,通过偏振分辨泵探针和 2D 红外光谱 (2D IR) 研究 N-甲基乙酰胺水溶液。将 2D IR 结果与分子动力学模拟和光谱计算进行比较,以深入了解混合物中的分子结构。N-甲基乙酰胺和水分子倾向于形成具有“冻结”酰胺 I 动力学的团簇。这是由 N-甲基乙酰胺分子的甲基在水存在下聚集时疏水塌陷驱动的。由于所研究的系统可以被认为是蛋白质骨架的简化模型,因此本研究为理解蛋白质的结构和振动动力学提供了方便的基础。特别有趣的是,在如此简单的系统中观察到作为驱动蛋白质折叠的疏水崩溃。
更新日期:2018-02-09
中文翻译:

N-甲基乙酰胺-水混合物中的疏水塌陷。
使用酰胺 I 模式作为报告基因,通过偏振分辨泵探针和 2D 红外光谱 (2D IR) 研究 N-甲基乙酰胺水溶液。将 2D IR 结果与分子动力学模拟和光谱计算进行比较,以深入了解混合物中的分子结构。N-甲基乙酰胺和水分子倾向于形成具有“冻结”酰胺 I 动力学的团簇。这是由 N-甲基乙酰胺分子的甲基在水存在下聚集时疏水塌陷驱动的。由于所研究的系统可以被认为是蛋白质骨架的简化模型,因此本研究为理解蛋白质的结构和振动动力学提供了方便的基础。特别有趣的是,在如此简单的系统中观察到作为驱动蛋白质折叠的疏水崩溃。






























 京公网安备 11010802027423号
京公网安备 11010802027423号