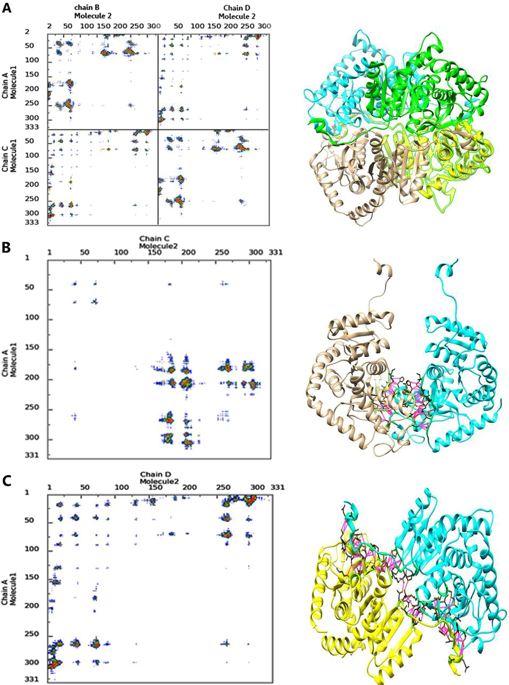
乳酸脱氢酶A(LDHA)是一种重要的代谢酶,属于2-羟基酸氧化还原酶家族,在细胞的厌氧代谢中起关键作用。在低氧条件下,LDHA的过表达将ATP合成的代谢途径从氧化磷酸化转变为有氧糖酵解,而低氧条件是肿瘤细胞微环境中的常见现象。因此,抑制LDHA被认为是癌症治疗的极佳策略。在这项研究中,我们聘用了计算机技术人员通过干扰酶四聚化来设计乳酸脱氢酶抑制肽的方法。使用肽作为抗癌剂是一种用于癌症治疗的新颖方法,相对于化学治疗药物具有一些优点,例如低毒性,易于合成和高靶标特异性。因此,肽可以与化合物并行地充当合适的酶抑制剂。在这项研究中,已采用多种计算技术,例如分子动力学(MD)模拟,对接和MM-PBSA计算来研究酶的单体,二聚体和四聚体形式的结构特征。MD模拟和蛋白质-蛋白质相互作用的分析表明,每个亚基的N末端臂在酶四聚化以建立酶的活性形式中具有重要作用。因此,N-末端臂可以用作肽设计的模板。然后,根据亲和力和理化性质对肽进行设计和评估,以获得最佳结合剂。最后,通过动态光散射(DLS)技术测量了肽对亚基缔合的抑制作用。我们的结果表明,设计的模拟酶N末端臂的肽可以成功靶向C末端结构域并打断酶亚基的真正形式。这项研究的结果为破坏组装过程提供了新途径,从而压制了LDHA的功能。N-末端臂可用作肽设计的模板。然后,根据亲和力和理化性质对肽进行设计和评估,以获得最佳结合剂。最后,通过动态光散射(DLS)技术测量了肽对亚基缔合的抑制作用。我们的结果表明,设计的模拟酶N末端臂的肽可以成功靶向C末端结构域并打断酶亚基的真正形式。这项研究的结果为破坏组装过程提供了新途径,从而压制了LDHA的功能。N-末端臂可用作肽设计的模板。然后,根据亲和力和理化性质对肽进行设计和评估,以获得最佳结合剂。最后,通过动态光散射(DLS)技术测量了肽对亚基缔合的抑制作用。我们的结果表明,设计的模拟酶N末端臂的肽可以成功靶向C末端结构域并打断酶亚基的真正形式。这项研究的结果为破坏组装过程提供了新途径,从而压制了LDHA的功能。通过动态光散射(DLS)技术测量了肽对亚基缔合的抑制作用。我们的结果表明,设计的模拟酶N末端臂的肽可以成功靶向C末端结构域并打断酶亚基的真正形式。这项研究的结果为破坏组装过程提供了新途径,从而压制了LDHA的功能。通过动态光散射(DLS)技术测量了肽对亚基缔合的抑制作用。我们的结果表明,设计的模拟酶N末端臂的肽可以成功靶向C末端结构域并打断酶亚基的真正形式。这项研究的结果为破坏组装过程提供了新途径,从而压制了LDHA的功能。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Novel Peptide Inhibitors for Lactate Dehydrogenase A (LDHA): A Survey to Inhibit LDHA Activity via Disruption of Protein-Protein Interaction
Lactate dehydrogenase A (LDHA) is a critical metabolic enzyme belonging to a family of 2-hydroxy acid oxidoreductases that plays a key role in anaerobic metabolism in the cells. In hypoxia condition, the overexpression of LDHA shifts the metabolic pathway of ATP synthesis from oxidative phosphorylation to aerobic glycolysis and the hypoxia condition is a common phenomenon occurred in the microenvironment of tumor cells; therefore, the inhibition of LDHA is considered to be an excellent strategy for cancer therapy. In this study, we employed in silico methods to design inhibitory peptides for lactate dehydrogenase through the disturbance in tetramerization of the enzyme. Using peptide as an anti-cancer agent is a novel approach for cancer therapy possessing some advantages with respect to the chemotherapeutic drugs such as low toxicity, ease of synthesis, and high target specificity. So peptides can act as appropriate enzyme inhibitor in parallel to chemical compounds. In this study, several computational techniques such as molecular dynamics (MD) simulation, docking and MM-PBSA calculation have been employed to investigate the structural characteristics of the monomer, dimer, and tetramer forms of the enzyme. Analysis of MD simulation and protein-protein interaction showed that the N-terminal arms of each subunit have an important role in enzyme tetramerization to establish active form of the enzyme. Hence, N-terminal arm can be used as a template for peptide design. Then, peptides were designed and evaluated to obtain best binders based on the affinity and physicochemical properties. Finally, the inhibitory effect of the peptides on subunit association was measured by dynamic light scattering (DLS) technique. Our results showed that the designed peptides which mimic the N-terminal arm of the enzyme can successfully target the C-terminal domain and interrupt the bona fide form of the enzyme subunits. The result of this study makes a new avenue to disrupt the assembly process and thereby oppress the function of the LDHA.


































 京公网安备 11010802027423号
京公网安备 11010802027423号