当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Separation and Complexation of Trivalent Actinide and Lanthanide by a Tetradentate Soft–Hard Donor Ligand: Solvent Extraction, Spectroscopy, and DFT Calculations
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-03-14 00:00:00 , DOI: 10.1021/acs.inorgchem.8b03592 Lei Xu 1 , Ning Pu 1 , Youzhen Li 1 , Pingping Wei 1 , Taoxiang Sun 1 , Chengliang Xiao 2 , Jing Chen 1 , Chao Xu 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-03-14 00:00:00 , DOI: 10.1021/acs.inorgchem.8b03592 Lei Xu 1 , Ning Pu 1 , Youzhen Li 1 , Pingping Wei 1 , Taoxiang Sun 1 , Chengliang Xiao 2 , Jing Chen 1 , Chao Xu 1
Affiliation
|
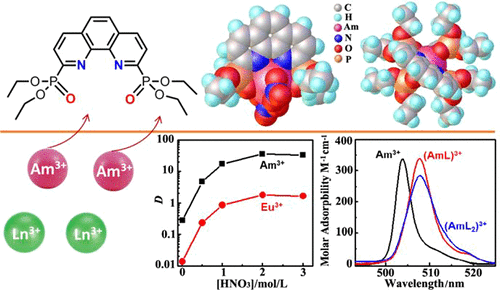
Recently, phenanthroline-based ligands have received increasing attention due to their excellent separation capabilities for trivalent actinides over lanthanide. In this work, we designed a soft–hard donor combined tetradentate phenanthroline-based extractant, tetraethyl (1,10-phenanthrolin-2,9-diyl)phosphonate (C2-POPhen), for the selective separation of trivalent Am(III) over Ln(III) from HNO3 media. The solvent extraction and complexation behaviors of Am(III) and Ln(III) by C2-POPhen were investigated both experimentally and theoretically. C2-POPhen could selectively extract Am(III) over Eu(III) with an extremely fast extraction kinetics. NMR titration studies suggest that only 1:1 complexes of Ln(III) with C2-POPhen formed in CH3OH in the presence of a significant amount of nitrate, while both 1:1 and 2:1 complexes species could form between C2-POPhen and Ln(III) perchlorate in CH3OH without nitrate ions. The stability constants for the complexation of Am(III) and Ln(III) with C2-POPhen in CH3OH were determined by spectrophotometric titrations and the Am(III) complexes are approximately 1 order of magnitude stronger than those of Ln(III), which is consistent with the extraction results. Theoretical calculations indicate that the Am–N bonds in Am/C2-POPhen complexes possess more covalent characters than the Eu–N bonds in Eu/C2-POPhen complexes, shedding light on the underlying chemical force responsible for the Am/Eu selectivity by C2-POPhen. This work represents the first report utilizing phenanthroline-based phosphonate ligands for selective separation of actinides from highly acidic solutions.
中文翻译:

四齿软硬施主配体选择性分离和络合三价Act系元素和镧系元素:溶剂萃取,光谱法和DFT计算
近来,基于菲咯啉的配体由于其对三价act系元素的优于镧系元素的出色分离能力而受到越来越多的关注。在这项工作中,我们设计了一种软-硬供体结合的基于四齿菲咯啉的萃取剂,四乙基(1,10-菲咯啉-2,9-二基)膦酸酯(C2-POPhen),用于选择性分离三价Am(III)。来自HNO 3介质的Ln(III)。通过实验和理论研究了C2-POPhen对Am(III)和Ln(III)的溶剂萃取和络合行为。C2-POPhen可以以极快的萃取动力学选择性地萃取Eu(III)上的Am(III)。NMR滴定研究表明,CH 3中仅形成了Ln(III)与C2-POPhen的1:1配合物OH存在大量硝酸盐时,在CH 2 OH中,没有硝酸根离子的情况下,C2-POPhen和高氯酸Ln(III)可能同时形成1:1和2:1的配合物。CH 3中Am(III)和Ln(III)与C2-POPhen络合的稳定常数OH是通过分光光度滴定法测定的,Am(III)络合物比Ln(III)的络合物强约1个数量级,这与萃取结果一致。理论计算表明,Am / C2-POPhen络合物中的Am-N键比Eu / C2-POPhen络合物中的Eu-N键具有更多的共价特征,从而阐明了由C2引起的Am / Eu选择性的潜在化学力。 -POPhen。这项工作代表了首次使用基于菲咯啉的膦酸酯配体从高酸性溶液中选择性分离act系元素的报告。
更新日期:2019-03-14
中文翻译:

四齿软硬施主配体选择性分离和络合三价Act系元素和镧系元素:溶剂萃取,光谱法和DFT计算
近来,基于菲咯啉的配体由于其对三价act系元素的优于镧系元素的出色分离能力而受到越来越多的关注。在这项工作中,我们设计了一种软-硬供体结合的基于四齿菲咯啉的萃取剂,四乙基(1,10-菲咯啉-2,9-二基)膦酸酯(C2-POPhen),用于选择性分离三价Am(III)。来自HNO 3介质的Ln(III)。通过实验和理论研究了C2-POPhen对Am(III)和Ln(III)的溶剂萃取和络合行为。C2-POPhen可以以极快的萃取动力学选择性地萃取Eu(III)上的Am(III)。NMR滴定研究表明,CH 3中仅形成了Ln(III)与C2-POPhen的1:1配合物OH存在大量硝酸盐时,在CH 2 OH中,没有硝酸根离子的情况下,C2-POPhen和高氯酸Ln(III)可能同时形成1:1和2:1的配合物。CH 3中Am(III)和Ln(III)与C2-POPhen络合的稳定常数OH是通过分光光度滴定法测定的,Am(III)络合物比Ln(III)的络合物强约1个数量级,这与萃取结果一致。理论计算表明,Am / C2-POPhen络合物中的Am-N键比Eu / C2-POPhen络合物中的Eu-N键具有更多的共价特征,从而阐明了由C2引起的Am / Eu选择性的潜在化学力。 -POPhen。这项工作代表了首次使用基于菲咯啉的膦酸酯配体从高酸性溶液中选择性分离act系元素的报告。































 京公网安备 11010802027423号
京公网安备 11010802027423号