Water Research ( IF 11.4 ) Pub Date : 2018-02-09 , DOI: 10.1016/j.watres.2018.02.008 Elodie Passeport , Ning Zhang , Langping Wu , Hartmut Herrmann , Barbara Sherwood Lollar , Hans-Hermann Richnow
|
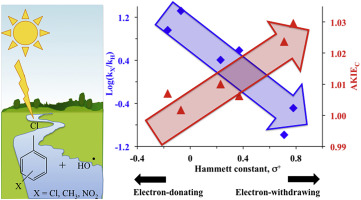
Substituted chlorobenzenes are the basic substructure of many surface water contaminants. In this study, the isotope fractionation and reaction mechanisms involved during the aqueous direct and indirect photodegradation of CH3-, Cl-, and NO2- substituted chlorobenzenes were investigated in laboratory experiments. Only 4-nitrochlorobenzene showed slow but isotopically fractionating direct photolysis. During indirect photodegradation using UV/H2O2-generated OH radicals, the pseudo first-order reaction rate constants increased in the order of the NO2- < Cl- < CH3- substituted chlorobenzenes. The most pronounced carbon enrichment factors were observed for nitrochlorobenzenes (up to −4.8 ± 0.5‰), whereas the least significant were for chlorotoluenes (≤−1.0 ± 0.1‰). As the substituents became more electron-withdrawing, the activation energy barrier increased, leading to slower reaction rates, and the transition state changed to a more symmetrical or less reactant-like structure, resulting in larger apparent kinetic isotope effects. The results suggest that the rate-determining step in the reaction with OH radicals was the addition of the electrophile to the benzene ring. Even though further research is needed to quantify isotope fractionation during other transformation processes, these results showed evidence that compound specific isotope analysis can be used as a diagnostic tool for the fate of substituted chlorobenzenes in water.
中文翻译:

取代氯苯的光降解:动力学,碳同位素分馏和反应机理
取代的氯苯是许多地表水污染物的基本子结构。在这项研究中,在实验室实验中研究了CH 3-,Cl-和NO 2-取代的氯苯在水中直接和间接光降解过程中涉及的同位素分馏和反应机理。仅4-硝基氯苯显示缓慢但同位素分馏的直接光解。在使用UV / H 2 O 2生成的OH自由基进行间接光降解过程中,伪一级反应速率常数按NO 2- <Cl- <CH 3的顺序增加。-取代的氯苯。硝基氯苯的碳富集系数最显着(最高-4.8±0.5‰),而氯甲苯的碳富集系数最低(≤-1.0±0.1‰)。随着取代基变得更加吸电子,活化能垒增加,导致反应速率降低,过渡态变为更对称或更不像反应物的结构,从而导致更大的表观动力学同位素效应。结果表明,与OH自由基反应的决定速率的步骤是将亲电子试剂加到苯环上。即使需要进一步研究来量化其他转化过程中的同位素分馏,











































 京公网安备 11010802027423号
京公网安备 11010802027423号