Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformation Dependence of Diphenylalanine Self-Assembly Structures and Dynamics: Insights from Hybrid-Resolution Simulations.
ACS Nano ( IF 15.8 ) Pub Date : 2019-03-14 00:00:00 , DOI: 10.1021/acsnano.8b09741 Qinsi Xiong 1 , Yixiang Jiang 1 , Xiang Cai 1 , Fadeng Yang 1 , Zigang Li 1 , Wei Han 1
ACS Nano ( IF 15.8 ) Pub Date : 2019-03-14 00:00:00 , DOI: 10.1021/acsnano.8b09741 Qinsi Xiong 1 , Yixiang Jiang 1 , Xiang Cai 1 , Fadeng Yang 1 , Zigang Li 1 , Wei Han 1
Affiliation
|
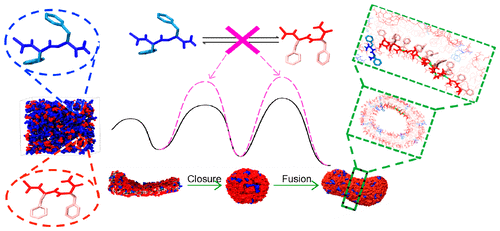
The molecular design of peptide-assembled nanostructures relies on extensive knowledge pertaining to the relationship between conformational features of peptide constituents and their behavior regarding self-assembly, and characterizing the conformational details of peptides during their self-assembly is experimentally challenging. Here, we demonstrate that a hybrid-resolution modeling method can be employed to investigate the role that conformation plays during the assembly of terminally capped diphenylalanines (FF) through microsecond simulations of hundreds or thousands of peptides. Our simulations discovered tubular or vesicular nanostructures that were consistent with experimental observation while reproducing critical self-assembly concentration and secondary structure contents in the assemblies that were measured in our experiments. The atomic details provided by our method allowed us to uncover diverse FF conformations and conformation dependence of assembled nanostructures. We found that the assembled morphologies and the molecular packing of FFs in the observed assemblies are linked closely with side-chain angle and peptide bond orientation, respectively. Of various conformations accessible to soluble FFs, only a select few are compatible with the assembled morphologies in water. A conformation resembling a FF crystal, in particular, became predominant due to its ability to permit highly ordered and energetically favorable FF packing in aqueous assemblies. Strikingly, several conformations incompatible with the assemblies arose transiently as intermediates, facilitating key steps of the assembly process. The molecular rationale behind the role of these intermediate conformations were further explained. Collectively, the structural details reported here advance the understanding of the FF self-assembly mechanism, and our method shows promise for studying peptide-assembled nanostructures and their rational design.
中文翻译:

构象依赖性的二苯丙氨酸自组装结构和动力学:混合分辨率模拟的见解。
肽组装的纳米结构的分子设计依赖于与肽成分的构象特征与其自组装行为之间的关系有关的广泛知识,表征自组装过程中肽的构象细节在实验上具有挑战性。在这里,我们证明了一种混合分辨率建模方法可以用来通过数百或数千个肽段的微秒模拟来研究构象在末端加帽的二苯丙氨酸(FF)组装过程中扮演的角色。我们的模拟发现与实验观察结果一致的管状或囊状纳米结构,同时在我们的实验中测量的组件中重现了关键的自组装浓度和二级结构含量。我们的方法提供的原子细节使我们能够发现各种FF构象和组装的纳米结构的构象依赖性。我们发现观察到的组件中的组装形态和FFs的分子堆积分别与侧链角和肽键方向紧密相连。在可溶性FF可获得的各种构型中,只有少数几个与水中组装的形态相容。特别地,由于其允许在水性组件中高度有序且在能量上有利的FF填充的能力,类似于FF晶体的构象变得占主导地位。引人注目的是,与装配体不兼容的几种构象作为中间体而短暂出现,从而促进了装配过程中的关键步骤。这些中间构象的作用背后的分子原理被进一步解释。总的来说,这里报道的结构细节促进了对FF自组装机制的理解,我们的方法显示了研究肽组装的纳米结构及其合理设计的希望。
更新日期:2019-03-14
中文翻译:

构象依赖性的二苯丙氨酸自组装结构和动力学:混合分辨率模拟的见解。
肽组装的纳米结构的分子设计依赖于与肽成分的构象特征与其自组装行为之间的关系有关的广泛知识,表征自组装过程中肽的构象细节在实验上具有挑战性。在这里,我们证明了一种混合分辨率建模方法可以用来通过数百或数千个肽段的微秒模拟来研究构象在末端加帽的二苯丙氨酸(FF)组装过程中扮演的角色。我们的模拟发现与实验观察结果一致的管状或囊状纳米结构,同时在我们的实验中测量的组件中重现了关键的自组装浓度和二级结构含量。我们的方法提供的原子细节使我们能够发现各种FF构象和组装的纳米结构的构象依赖性。我们发现观察到的组件中的组装形态和FFs的分子堆积分别与侧链角和肽键方向紧密相连。在可溶性FF可获得的各种构型中,只有少数几个与水中组装的形态相容。特别地,由于其允许在水性组件中高度有序且在能量上有利的FF填充的能力,类似于FF晶体的构象变得占主导地位。引人注目的是,与装配体不兼容的几种构象作为中间体而短暂出现,从而促进了装配过程中的关键步骤。这些中间构象的作用背后的分子原理被进一步解释。总的来说,这里报道的结构细节促进了对FF自组装机制的理解,我们的方法显示了研究肽组装的纳米结构及其合理设计的希望。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号