当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of (−)‐Cephalotaxine and (−)‐Homoharringtonine via Furan Oxidation–Transannular Mannich Cyclization
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-04-04 , DOI: 10.1002/anie.201902174 Xuan Ju 1 , Christopher M. Beaudry 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-04-04 , DOI: 10.1002/anie.201902174 Xuan Ju 1 , Christopher M. Beaudry 1
Affiliation
|
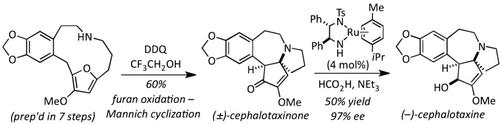
Homoharringtonine and its congener cephalotaxine were synthesized. Oxidative ring‐opening of a furan unveils an amine‐tethered dicarbonyl, which undergoes spontaneous transannular Mannich cyclization. The cascade builds the full cephalotaxine substructure in a single operation in 60 % yield. A Noyori reduction enabled synthesis of the title compounds with excellent enantioselectivity (krel=278).
中文翻译:

通过呋喃氧化-环过曼尼希环化法合成(-)-头孢他辛和(-)-高灵碱
合成了人harringtonine及其同类物头孢他辛。呋喃的氧化性开环揭示了一种胺系的二羰基化合物,该胺基化合物会自发地经过环面Mannich环化。级联通过一次操作以60%的产率构建完整的头孢他辛亚结构。通过Noyori还原,能够以优异的对映选择性(k rel= 278)合成标题化合物。
更新日期:2019-04-04
中文翻译:

通过呋喃氧化-环过曼尼希环化法合成(-)-头孢他辛和(-)-高灵碱
合成了人harringtonine及其同类物头孢他辛。呋喃的氧化性开环揭示了一种胺系的二羰基化合物,该胺基化合物会自发地经过环面Mannich环化。级联通过一次操作以60%的产率构建完整的头孢他辛亚结构。通过Noyori还原,能够以优异的对映选择性(k rel= 278)合成标题化合物。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号