当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, synthesis and bioevaluation of 3-oxo-6-aryl-2,3-dihydropyridazine-4-carbohydrazide derivatives as novel xanthine oxidase inhibitors.
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-03-13 , DOI: 10.1016/j.bmc.2019.03.027 Lichao Zhang 1 , Sibo Wang 1 , Mingzheng Yang 2 , Ailong Shi 3 , He Wang 3 , Qi Guan 2 , Kai Bao 1 , Weige Zhang 3
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-03-13 , DOI: 10.1016/j.bmc.2019.03.027 Lichao Zhang 1 , Sibo Wang 1 , Mingzheng Yang 2 , Ailong Shi 3 , He Wang 3 , Qi Guan 2 , Kai Bao 1 , Weige Zhang 3
Affiliation
|
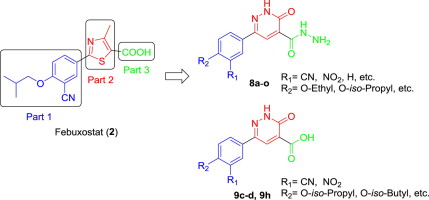
In view of expanding the structure activity relationship of xanthine oxidase inhibitors, a series of 3-oxo-6-aryl-2,3-dihydropyridazine-4-carbohydrazide/carboxylic acid derivatives were designed by molecular docking and synthesized. All the target compounds were evaluated for their in vitro XO inhibition by using febuxostat and allopurinol as the standard controls. Most of the hydrazide derivatives exhibited potency levels in the micromolar range. From the view of docking study, hydrazide derivatives bind to the active site of XO through a novel interaction mode, which is different from that of febuxostat bearing a carboxyl group. The most promising compound 8b was further subjected to kinetic analysis to deduce their modes of inhibition.
中文翻译:

设计,合成和生物评价3-氧代-6-芳基-2,3-二氢哒嗪-4-碳酰肼衍生物作为新型黄嘌呤氧化酶抑制剂。
为了扩大黄嘌呤氧化酶抑制剂的结构活性关系,通过分子对接设计合成了一系列3-氧代-6-芳基-2,3-二氢哒嗪-4-羧酰肼/羧酸衍生物。使用非布索坦和别嘌呤醇作为标准对照,评估所有目标化合物的体外XO抑制作用。大多数酰肼衍生物表现出在微摩尔范围内的效力水平。从对接研究的角度来看,酰肼衍生物通过一种新颖的相互作用方式与XO的活性位点结合,这与带有羧基的非布索坦的相互作用方式不同。最有希望的化合物8b进一步进行动力学分析,以推论其抑制方式。
更新日期:2019-03-13
中文翻译:

设计,合成和生物评价3-氧代-6-芳基-2,3-二氢哒嗪-4-碳酰肼衍生物作为新型黄嘌呤氧化酶抑制剂。
为了扩大黄嘌呤氧化酶抑制剂的结构活性关系,通过分子对接设计合成了一系列3-氧代-6-芳基-2,3-二氢哒嗪-4-羧酰肼/羧酸衍生物。使用非布索坦和别嘌呤醇作为标准对照,评估所有目标化合物的体外XO抑制作用。大多数酰肼衍生物表现出在微摩尔范围内的效力水平。从对接研究的角度来看,酰肼衍生物通过一种新颖的相互作用方式与XO的活性位点结合,这与带有羧基的非布索坦的相互作用方式不同。最有希望的化合物8b进一步进行动力学分析,以推论其抑制方式。














































 京公网安备 11010802027423号
京公网安备 11010802027423号