当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
LC-MS/MS assay for the determination of a novel anti-fibrotic candidate mefunidone in monkey plasma and its application to a pharmacokinetics study.
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2019-04-15 , DOI: 10.1002/dta.2588 Xuhua Han 1 , Zhou Wen 1, 2 , Zhihong Fan 3 , Yuehui Ma 3 , Lei Wang 1, 4 , Zeneng Cheng 1
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2019-04-15 , DOI: 10.1002/dta.2588 Xuhua Han 1 , Zhou Wen 1, 2 , Zhihong Fan 3 , Yuehui Ma 3 , Lei Wang 1, 4 , Zeneng Cheng 1
Affiliation
|
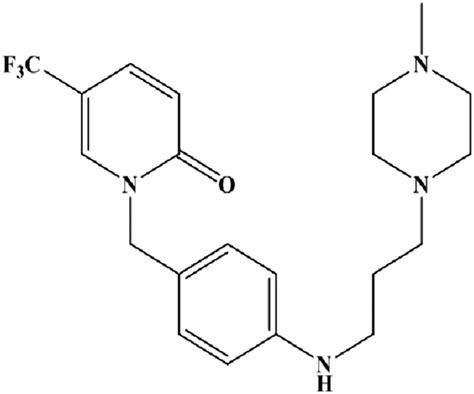
Mefunidone (MFD) is a promising anti‐fibrotic candidate molecule with greater anti‐fibrotic activity than pirfenidone (PFD). However, there has been no report on the methodology used for the quantification of MFD or on any investigation of its pharmacokinetics. In this study, an efficient and reliable liquid chromatography–tandem mass spectrometry (LC–MS/MS) method was developed to assay MFD in monkey plasma. This assay method was validated and applied to a pharmacokinetics study in monkeys. The lower limit of quantification of this assay was 0.1 μg·mL−1, and the linear calibration curve was acquired with R2 > 0.99 between 0.1 and 60 μg·mL−1. The intra‐day and inter‐day precision were evaluated with coefficient of variations of 1.5%–5.8%, whereas the mean accuracy ranged from 91.7% to 106.9%. A negligible matrix effect and good recovery were obtained using this assay, with average extraction recoveries of MFD and the internal standard (IS) in the range of 85.5%–124.8% and 84.1%–94.0%, respectively. The precision of the absolute matrix effect of MFD and the IS was 1.2–3.0% and 1.2–7.3%, respectively. The samples were stable under all experimental conditions. Linear pharmacokinetics were observed for MFD in monkeys, where the exposures of MFD increased proportionally with increasing MFD doses at the range of 10–90 mg·kg−1. Moderate elimination of MFD from the body was observed, with t1/2 of 5–7 h, and the elimination rate of MFD was stable during multiple dosing. In conclusion, this method provides an reliable analytical approach for quantification of MFD in plasma and was successfully applied to a pharmacokinetics study in monkeys.
中文翻译:

LC-MS / MS法测定猴血浆中新型抗纤维化候选药物美非尼酮及其在药代动力学研究中的应用。
美非尼酮(MFD)是一种有希望的抗纤维化候选分子,其抗纤维化活性高于吡非尼酮(PFD)。但是,目前尚无关于MFD定量方法或其药代动力学研究的报告。在这项研究中,开发了一种高效可靠的液相色谱-串联质谱(LC-MS / MS)方法来测定猴血浆中的MFD。验证了该测定方法,并将其应用于猴子的药代动力学研究。该测定的定量下限为0.1μg·mL -1,并且线性校准曲线的获得 为0.1至60μg·mL -1之间的R 2 > 0.99。评估日内和日间精度的变异系数为1.5%-5.8%,而平均精度范围为91.7%至106.9%。使用该检测方法可获得的基质效应和回收率均可以忽略不计,MFD和内标物(IS)的平均提取回收率分别为85.5%–124.8%和84.1%–94.0%。MFD和IS的绝对矩阵效应的精度分别为1.2–3.0%和1.2–7.3%。样品在所有实验条件下均稳定。在猴子中,MFD的线性药代动力学被观察到,在10–90 mg·kg -1的范围内,MFD的暴露与MFD剂量的增加成比例地增加。观察到人体中MFD的中度消除,t 1/2在5-7小时内,MFD的消除率在多次给药过程中保持稳定。总之,该方法为定量血浆中的MFD提供了可靠的分析方法,并已成功应用于猴子的药代动力学研究。
更新日期:2019-04-15
中文翻译:

LC-MS / MS法测定猴血浆中新型抗纤维化候选药物美非尼酮及其在药代动力学研究中的应用。
美非尼酮(MFD)是一种有希望的抗纤维化候选分子,其抗纤维化活性高于吡非尼酮(PFD)。但是,目前尚无关于MFD定量方法或其药代动力学研究的报告。在这项研究中,开发了一种高效可靠的液相色谱-串联质谱(LC-MS / MS)方法来测定猴血浆中的MFD。验证了该测定方法,并将其应用于猴子的药代动力学研究。该测定的定量下限为0.1μg·mL -1,并且线性校准曲线的获得 为0.1至60μg·mL -1之间的R 2 > 0.99。评估日内和日间精度的变异系数为1.5%-5.8%,而平均精度范围为91.7%至106.9%。使用该检测方法可获得的基质效应和回收率均可以忽略不计,MFD和内标物(IS)的平均提取回收率分别为85.5%–124.8%和84.1%–94.0%。MFD和IS的绝对矩阵效应的精度分别为1.2–3.0%和1.2–7.3%。样品在所有实验条件下均稳定。在猴子中,MFD的线性药代动力学被观察到,在10–90 mg·kg -1的范围内,MFD的暴露与MFD剂量的增加成比例地增加。观察到人体中MFD的中度消除,t 1/2在5-7小时内,MFD的消除率在多次给药过程中保持稳定。总之,该方法为定量血浆中的MFD提供了可靠的分析方法,并已成功应用于猴子的药代动力学研究。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号