当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Geometric Occupancy and Oxidation State Requirements of Cations in Cobalt Oxides for Oxygen Reduction Reaction
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-03-14 00:00:00 , DOI: 10.1021/acsami.9b00481 Jing Liu 1 , Hongliang Bao 2 , Bingsen Zhang 3 , Qingfeng Hua 1 , Mingfeng Shang 2 , Jianqiang Wang 2 , Luhua Jiang 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-03-14 00:00:00 , DOI: 10.1021/acsami.9b00481 Jing Liu 1 , Hongliang Bao 2 , Bingsen Zhang 3 , Qingfeng Hua 1 , Mingfeng Shang 2 , Jianqiang Wang 2 , Luhua Jiang 1
Affiliation
|
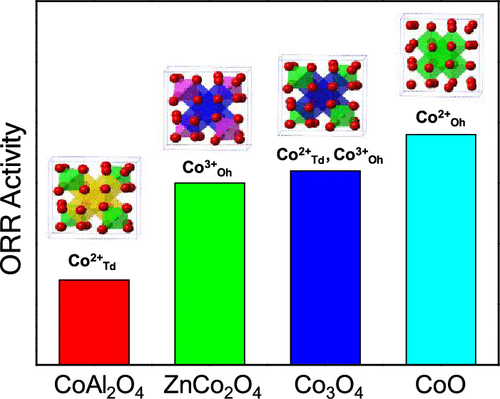
Cobalt oxides, including spinel Co3O4 and rock-salt CoO, have been widely reported as promising catalysts for oxygen reduction reaction (ORR). However, three types of cobalt ions, i.e., Co2+ in the tetrahedral site (Co2+Td), Co3+ in the octahedral site (Co3+Oh), and Co2+ in the octahedral site (Co2+Oh), are included in these oxides, and the roles of cobalt geometric occupancy and valance states have remained elusive. Here, for the first time, we investigated the effects of cobalt geometric occupancy on the ORR activity by substituting Co2+Td and Co3+Oh of Co3O4 with inactive Zn2+ and Al3+, respectively. The ORR activity decreases in the order of Co3O4 (Co3+Oh, Co2+Td) < ZnCo2O4 (Co3+Oh) ≪ CoAl2O4 (Co2+Td) in accordance with the ORR overpotentials at the current density of 0.1 mA cmOx–2. Furthermore, by comparatively investigating the activity and stability of Co3O4 (Co3+Oh) and CoO (Co2+Oh) nanoparticles, by virtue of the electrochemical technique, the high-resolution transmission electron microscopy, and the in operando fuel cell–X-ray absorption spectroscopy techniques, it was revealed that Co2+Oh in CoO is the main active site, which under electrochemical conditions tends to transform into Co3+Oh and form Co3O4 with a hollow structure due to the Kirkendall effect; nevertheless, it retains decent ORR activity due to the formation of the unique hollow structure.
中文翻译:

氧化还原反应中氧化钴中阳离子的几何占有率和氧化态要求
包括尖晶石Co 3 O 4和岩盐CoO在内的氧化钴已被广泛报道为氧还原反应(ORR)的有前途的催化剂。然而,三种类型的钴离子,即,钴2+在四面体位点(共2+ Ť d),钴3+在八面体位置(钴3+ Ò ħ)和Co 2+在八面体位置(合这些氧化物中包含2+ O h),并且钴几何占有率和价态的作用仍然难以捉摸。在这里,我们首次通过取代Co 2+来研究钴的几何占有率对ORR活性的影响。具有惰性Zn 2+和Al 3+的Co 3 O 4的T d和Co 3+ O h分别为。ORR活性按Co 3 O 4(Co 3+ O h,Co 2+ T d)<ZnCo 2 O 4(Co 3+ O h)≪ CoAl 2 O 4(Co 2+ T d)的顺序降低。在电流密度为0.1 mA cm Ox –2时符合ORR过电势。此外,通过电化学技术,高分辨率透射电子显微镜和红外光谱,对Co 3 O 4(Co 3+ O h)和CoO(Co 2+ O h)纳米粒子的活性和稳定性进行了比较研究。操作燃料电池-X射线吸收光谱技术表明,CoO中的Co 2+ O h是主要的活性位点,在电化学条件下倾向于转化为Co 3+ O h并形成Co 3 O 4。由于柯肯德尔效应而具有空心结构;然而,由于独特的中空结构的形成,它保留了不错的ORR活性。
更新日期:2019-03-14
中文翻译:

氧化还原反应中氧化钴中阳离子的几何占有率和氧化态要求
包括尖晶石Co 3 O 4和岩盐CoO在内的氧化钴已被广泛报道为氧还原反应(ORR)的有前途的催化剂。然而,三种类型的钴离子,即,钴2+在四面体位点(共2+ Ť d),钴3+在八面体位置(钴3+ Ò ħ)和Co 2+在八面体位置(合这些氧化物中包含2+ O h),并且钴几何占有率和价态的作用仍然难以捉摸。在这里,我们首次通过取代Co 2+来研究钴的几何占有率对ORR活性的影响。具有惰性Zn 2+和Al 3+的Co 3 O 4的T d和Co 3+ O h分别为。ORR活性按Co 3 O 4(Co 3+ O h,Co 2+ T d)<ZnCo 2 O 4(Co 3+ O h)≪ CoAl 2 O 4(Co 2+ T d)的顺序降低。在电流密度为0.1 mA cm Ox –2时符合ORR过电势。此外,通过电化学技术,高分辨率透射电子显微镜和红外光谱,对Co 3 O 4(Co 3+ O h)和CoO(Co 2+ O h)纳米粒子的活性和稳定性进行了比较研究。操作燃料电池-X射线吸收光谱技术表明,CoO中的Co 2+ O h是主要的活性位点,在电化学条件下倾向于转化为Co 3+ O h并形成Co 3 O 4。由于柯肯德尔效应而具有空心结构;然而,由于独特的中空结构的形成,它保留了不错的ORR活性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号