当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Upshift of the d Band Center toward the Fermi Level for Promoting Silver Ion Release, Bacteria Inactivation, and Wound Healing of Alloy Silver Nanoparticles
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-03-13 00:00:00 , DOI: 10.1021/acsami.8b21768 Yun Chang 1 , Yan Cheng , Yanlin Feng 2 , Kai Li , Hui Jian , Haiyuan Zhang 1, 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-03-13 00:00:00 , DOI: 10.1021/acsami.8b21768 Yun Chang 1 , Yan Cheng , Yanlin Feng 2 , Kai Li , Hui Jian , Haiyuan Zhang 1, 2
Affiliation
|
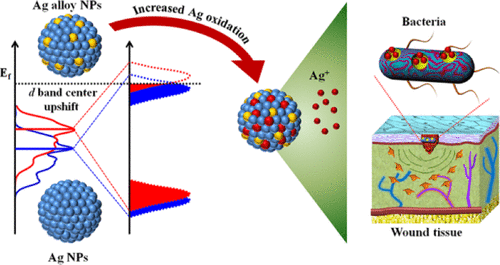
Silver (Ag)-based nanoparticles (NPs) with a high potential of Ag+ release have been known to be capable of promoting bacteria inactivation and the wound healing process; however, keeping a steady flux of high levels of Ag+ in Ag-based NPs is still challenging. Herein, a novel strategy in terms of altering the intrinsic electronic structure of Ag NPs was attempted to facilitate Ag oxidation and boost the Ag+ flux, as results of improved antibacterial and wound healing performance of Ag NPs. Gold (Au), platinum (Pt), and palladium (Pd) were doped into Ag NPs to tune their d band centers to upshift toward the Fermi level, and the formed Pd–Ag alloy NPs showed the largest shift, followed by Pt–Ag and Au–Ag NPs, as determined by density function theory calculation and ultraviolet photoemission spectroscopy measurement. Further X-ray photoelectron spectroscopy analysis indicates that a larger upshift could induce less electron filling in the antibonding orbital and a higher Ag oxidation level, leading to the more remarkable Ag+ release as determined by inductively coupled plasma optical emission spectrometry. All these alloy Ag NPs could more efficiently inhibit bacterial growth and accelerate the wound healing process than pure Ag NPs, and their antibacterial activity and wound healing performance were progressively proportional to the upshift values of the d band center. Taken together, tuning the d band center to upshift toward the Fermi level becomes a feasible strategy for designing therapeutic Ag-based NPs with a promising antibacterial and wound healing performance.
中文翻译:

d带中心向费米能级上移,以促进银离子释放,细菌失活和合金银纳米颗粒的伤口愈合
已知具有高释放Ag +潜力的基于银(Ag)的纳米颗粒(NPs)能够促进细菌失活和伤口愈合过程。然而,在基于Ag的NP中保持高水平的Ag +稳定流量仍是一项挑战。在本文中,尝试了一种改变Ag NPs固有电子结构的新策略,以促进Ag氧化并提高Ag +Ag NPs的抗菌和伤口愈合性能得到改善的结果。将金(Au),铂(Pt)和钯(Pd)掺入Ag NP中以调整其d能带中心,使其向费米能级上移,并且形成的Pd-Ag合金NPs表现出最大的偏移,其次是Pt- Ag和Au-Ag NPs,由密度函数理论计算和紫外光发射光谱法测量确定。进一步的X射线光电子能谱分析表明,较大的升档可以在反键轨道上引起较少的电子填充,并具有较高的Ag氧化水平,从而导致更显着的Ag +如通过电感耦合等离子体光学发射光谱法所确定的释放。所有这些合金Ag NPs均比纯Ag NPs更有效地抑制细菌生长并加速伤口愈合过程,并且它们的抗菌活性和伤口愈合性能与d带中心的上移值逐渐成比例。综上所述,调整d波段中心以向费米水平上移已成为设计具有前景的抗菌和伤口愈合性能的基于治疗的基于银的NP的可行策略。
更新日期:2019-03-13
中文翻译:

d带中心向费米能级上移,以促进银离子释放,细菌失活和合金银纳米颗粒的伤口愈合
已知具有高释放Ag +潜力的基于银(Ag)的纳米颗粒(NPs)能够促进细菌失活和伤口愈合过程。然而,在基于Ag的NP中保持高水平的Ag +稳定流量仍是一项挑战。在本文中,尝试了一种改变Ag NPs固有电子结构的新策略,以促进Ag氧化并提高Ag +Ag NPs的抗菌和伤口愈合性能得到改善的结果。将金(Au),铂(Pt)和钯(Pd)掺入Ag NP中以调整其d能带中心,使其向费米能级上移,并且形成的Pd-Ag合金NPs表现出最大的偏移,其次是Pt- Ag和Au-Ag NPs,由密度函数理论计算和紫外光发射光谱法测量确定。进一步的X射线光电子能谱分析表明,较大的升档可以在反键轨道上引起较少的电子填充,并具有较高的Ag氧化水平,从而导致更显着的Ag +如通过电感耦合等离子体光学发射光谱法所确定的释放。所有这些合金Ag NPs均比纯Ag NPs更有效地抑制细菌生长并加速伤口愈合过程,并且它们的抗菌活性和伤口愈合性能与d带中心的上移值逐渐成比例。综上所述,调整d波段中心以向费米水平上移已成为设计具有前景的抗菌和伤口愈合性能的基于治疗的基于银的NP的可行策略。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号