当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Unified Explanation for Chemoselectivity and Stereospecificity of Ni-Catalyzed Kumada and Cross-Electrophile Coupling Reactions of Benzylic Ethers: A Combined Computational and Experimental Study
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-03-13 , DOI: 10.1021/jacs.9b00097 Pan-Pan Chen 1 , Erika L Lucas 2 , Margaret A Greene 2 , Shuo-Qing Zhang 1 , Emily J Tollefson 2 , Lucas W Erickson 2 , Buck L H Taylor 3 , Elizabeth R Jarvo 2 , Xin Hong 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-03-13 , DOI: 10.1021/jacs.9b00097 Pan-Pan Chen 1 , Erika L Lucas 2 , Margaret A Greene 2 , Shuo-Qing Zhang 1 , Emily J Tollefson 2 , Lucas W Erickson 2 , Buck L H Taylor 3 , Elizabeth R Jarvo 2 , Xin Hong 1
Affiliation
|
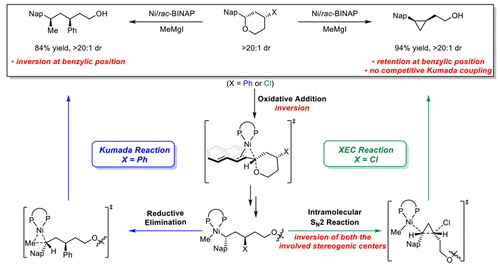
Ni-catalyzed C(sp3)-O bond activation provides a useful approach to synthesize enantioenriched products from readily available enantioenriched benzylic alcohol derivatives. The control of stereospecificity is key to the success of these transformations. To elucidate the reversed stereospecificity and chemoselectivity of Ni-catalyzed Kumada and cross-electrophile coupling reactions with benzylic ethers, a combined computational and experimental study is performed to reach a unified mechanistic understanding. Kumada coupling proceeds via a classic cross-coupling mechanism. Initial rate-determining oxidative addition occurs with stereoinversion of the benzylic stereogenic center. Subsequent transmetalation with the Grignard reagent and syn-reductive elimination produce the Kumada coupling product with overall stereoinversion at the benzylic position. The cross-electrophile coupling reaction initiates with the same benzylic C-O bond cleavage and transmetalation to form a common benzylnickel intermediate. However, the presence of the tethered alkyl chloride allows a facile intramolecular SN2 attack by the benzylnickel moiety. This step circumvents the competing Kumada coupling, leading to the excellent chemoselectivity of cross-electrophile coupling. These mechanisms account for the observed stereospecificity of the Kumada and cross-electrophile couplings, providing a rationale for double inversion of the benzylic stereogenic center in cross-electrophile coupling. The improved mechanistic understanding will enable design of stereoselective transformations involving Ni-catalyzed C(sp3)-O bond activation.
中文翻译:

Ni催化熊田的化学选择性和立体特异性的统一解释和苄醚的交叉电子偶联反应:计算和实验相结合的研究
Ni 催化的 C(sp3)-O 键活化提供了一种有用的方法来从容易获得的对映体富集的苯甲醇衍生物合成对映体富集的产品。立体特异性的控制是这些转化成功的关键。为了阐明 Ni 催化的熊田和与苄醚的交叉亲电偶联反应的反向立体特异性和化学选择性,进行了计算和实验相结合的研究,以达成统一的机理理解。熊田耦合通过经典的交叉耦合机制进行。初始速率决定氧化加成伴随苄基立体中心的立体反转发生。随后用格氏试剂进行金属转移和顺式还原消除产生熊田偶联产物,在苄基位置具有整体立体反转。交叉亲电偶联反应以相同的苄基 CO 键裂解和金属转移开始,以形成常见的苄基镍中间体。然而,系链烷基氯的存在允许苄基镍部分轻松进行分子内 SN2 攻击。这一步避免了竞争性的熊田偶联,导致交叉亲电偶联的优异化学选择性。这些机制解释了观察到的熊田和交叉亲电偶联的立体特异性,为交叉亲电偶联中苄基立体中心的双重反转提供了基本原理。改进的机械理解将能够设计涉及 Ni 催化的 C(sp3)-O 键活化的立体选择性转换。
更新日期:2019-03-13
中文翻译:

Ni催化熊田的化学选择性和立体特异性的统一解释和苄醚的交叉电子偶联反应:计算和实验相结合的研究
Ni 催化的 C(sp3)-O 键活化提供了一种有用的方法来从容易获得的对映体富集的苯甲醇衍生物合成对映体富集的产品。立体特异性的控制是这些转化成功的关键。为了阐明 Ni 催化的熊田和与苄醚的交叉亲电偶联反应的反向立体特异性和化学选择性,进行了计算和实验相结合的研究,以达成统一的机理理解。熊田耦合通过经典的交叉耦合机制进行。初始速率决定氧化加成伴随苄基立体中心的立体反转发生。随后用格氏试剂进行金属转移和顺式还原消除产生熊田偶联产物,在苄基位置具有整体立体反转。交叉亲电偶联反应以相同的苄基 CO 键裂解和金属转移开始,以形成常见的苄基镍中间体。然而,系链烷基氯的存在允许苄基镍部分轻松进行分子内 SN2 攻击。这一步避免了竞争性的熊田偶联,导致交叉亲电偶联的优异化学选择性。这些机制解释了观察到的熊田和交叉亲电偶联的立体特异性,为交叉亲电偶联中苄基立体中心的双重反转提供了基本原理。改进的机械理解将能够设计涉及 Ni 催化的 C(sp3)-O 键活化的立体选择性转换。















































 京公网安备 11010802027423号
京公网安备 11010802027423号