Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A concentrated poly(ethylene carbonate)/poly(trimethylene carbonate) blend electrolyte for all-solid-state Li battery
Polymer Journal ( IF 2.3 ) Pub Date : 2019-03-12 , DOI: 10.1038/s41428-019-0184-5 Zhenguang Li , Jonas Mindemark , Daniel Brandell , Yoichi Tominaga
Polymer Journal ( IF 2.3 ) Pub Date : 2019-03-12 , DOI: 10.1038/s41428-019-0184-5 Zhenguang Li , Jonas Mindemark , Daniel Brandell , Yoichi Tominaga
|
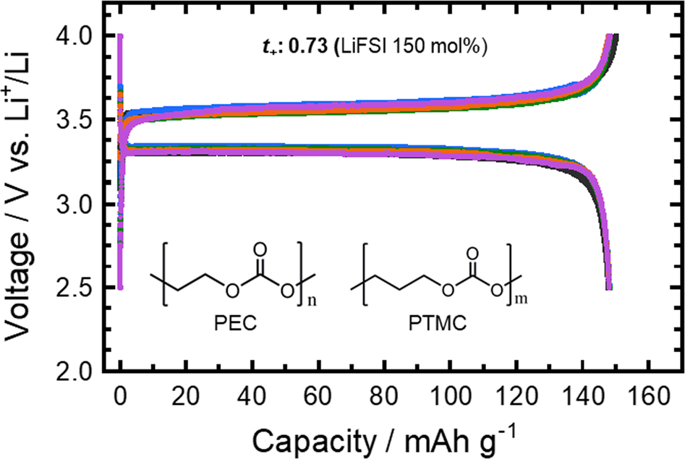
Electrochemical and ion-transport properties of polymer blend electrolytes comprising poly(ethylene carbonate) (PEC), poly(trimethylene carbonate) (PTMC) and lithium bis(fluorosulfonyl) imide (LiFSI) were studied in this work, and the electrolyte with the best blend composition was applied in all-solid-state Li batteries. The ionic conductivity of both PEC and PTMC single-polymer electrolytes increased with increasing Li salt concentration. All PEC and PTMC blend electrolytes show ionic conductivities on the order of 10−5 S cm−1 at 50 °C, and the ionic conductivities increase slightly with increasing PEC contents. The PEC6PTMC4-LiFSI 150 mol% electrolyte demonstrated better Li/electrolyte electrochemical and interfacial stability than that of PEC and PTMC single-polymer electrolytes and maintained a polarization as low as 5 mV for up to 200 h during Li metal plating and stripping. A Li|SPE|LFP cell with the PEC6PTMC4-LiFSI 150 mol% electrolyte exhibited reversible charge/discharge capacities close to 150 mAh g−1 at 50 °C and a C/10 rate, which is 88% of the theoretical value (170 mAh g−1).Concentrated poly(ethylene carbonate) (PEC) and poly(trimethylene carbonate) (PTMC) blend electrolytes with 150 mol% of LiFSI were prepared and the PEC6PTMC4 electrolyte was applied in all-solid-state Li batteries. The blend electrolyte has an ionic conductivity of 10−5 S cm−1 and a Li+ transference number (t+) of 0.73. Meanwhile, the PEC6PTMC4 electrolyte exhibits a good electrochemical stability with Li electrode than those of PEC and PTMC single-polymer electrolytes. An LFP half-cell exhibits a discharge capacity of 150 mAh g−1 at 50 °C and a C/10 rate.
中文翻译:

用于全固态锂电池的浓缩聚(碳酸亚乙酯)/聚(碳酸亚乙酯)混合电解质
本工作研究了包含聚碳酸亚乙酯 (PEC)、聚碳酸亚丙酯 (PTMC) 和双(氟磺酰基) 亚胺锂 (LiFSI) 的聚合物共混电解质的电化学和离子传输性能,并研究了具有最佳性能的电解质。共混组合物应用于全固态锂电池。PEC 和 PTMC 单聚合物电解质的离子电导率随着锂盐浓度的增加而增加。所有 PEC 和 PTMC 混合电解质在 50°C 下都显示出大约 10-5 S cm-1 的离子电导率,并且离子电导率随着 PEC 含量的增加而略有增加。PEC6PTMC4-LiFSI 150 mol% 电解质表现出比 PEC 和 PTMC 单聚合物电解质更好的锂/电解质电化学和界面稳定性,并在锂金属电镀和剥离过程中保持低至 5 mV 的极化长达 200 小时。具有 PEC6PTMC4-LiFSI 150 mol% 电解质的 Li|SPE|LFP 电池在 50 °C 和 C/10 倍率下表现出接近 150 mAh g-1 的可逆充电/放电容量,是理论值 (170 mAh g-1). 制备了含 150 mol% LiFSI 的浓聚碳酸亚乙酯 (PEC) 和聚碳酸亚丙酯 (PTMC) 混合电解质,并将 PEC6PTMC4 电解质应用于全固态锂电池。混合电解质的离子电导率为 10-5 S cm-1,Li+ 迁移数 (t+) 为 0.73。同时,与 PEC 和 PTMC 单聚合物电解质相比,PEC6PTMC4 电解质对锂电极表现出良好的电化学稳定性。LFP 半电池在 50°C 和 C/10 倍率下的放电容量为 150 mAh g-1。
更新日期:2019-03-12
中文翻译:

用于全固态锂电池的浓缩聚(碳酸亚乙酯)/聚(碳酸亚乙酯)混合电解质
本工作研究了包含聚碳酸亚乙酯 (PEC)、聚碳酸亚丙酯 (PTMC) 和双(氟磺酰基) 亚胺锂 (LiFSI) 的聚合物共混电解质的电化学和离子传输性能,并研究了具有最佳性能的电解质。共混组合物应用于全固态锂电池。PEC 和 PTMC 单聚合物电解质的离子电导率随着锂盐浓度的增加而增加。所有 PEC 和 PTMC 混合电解质在 50°C 下都显示出大约 10-5 S cm-1 的离子电导率,并且离子电导率随着 PEC 含量的增加而略有增加。PEC6PTMC4-LiFSI 150 mol% 电解质表现出比 PEC 和 PTMC 单聚合物电解质更好的锂/电解质电化学和界面稳定性,并在锂金属电镀和剥离过程中保持低至 5 mV 的极化长达 200 小时。具有 PEC6PTMC4-LiFSI 150 mol% 电解质的 Li|SPE|LFP 电池在 50 °C 和 C/10 倍率下表现出接近 150 mAh g-1 的可逆充电/放电容量,是理论值 (170 mAh g-1). 制备了含 150 mol% LiFSI 的浓聚碳酸亚乙酯 (PEC) 和聚碳酸亚丙酯 (PTMC) 混合电解质,并将 PEC6PTMC4 电解质应用于全固态锂电池。混合电解质的离子电导率为 10-5 S cm-1,Li+ 迁移数 (t+) 为 0.73。同时,与 PEC 和 PTMC 单聚合物电解质相比,PEC6PTMC4 电解质对锂电极表现出良好的电化学稳定性。LFP 半电池在 50°C 和 C/10 倍率下的放电容量为 150 mAh g-1。

































 京公网安备 11010802027423号
京公网安备 11010802027423号