当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
H2 Generation from H2O and H2S through an Iodine Cycle
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-03-13 00:00:00 , DOI: 10.1021/acssuschemeng.9b00600
Ryan J. Gillis 1 , Phalgun Lolur 1 , William H. Green 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-03-13 00:00:00 , DOI: 10.1021/acssuschemeng.9b00600
Ryan J. Gillis 1 , Phalgun Lolur 1 , William H. Green 1
Affiliation
|
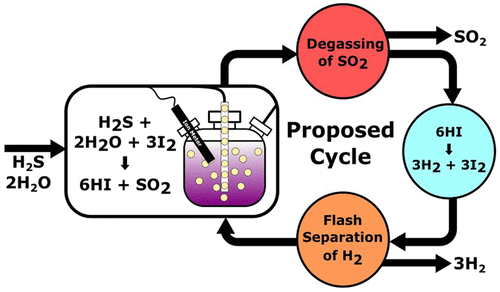
During an investigation of thermochemical methods of H2 generation from H2S, we observed the formation of unexpected products according to the reaction, H2S + 3I2 + 2H2O → 6HI + SO2. When coupled with the well-known catalyzed decomposition, 2HI → H2 + I2, this enables the formation of 3H2 per H2S reacted according to the reaction, H2S + 2H2O → 3H2 + SO2. Restated, these two reactions together accomplish the splitting of H2O driven by the oxidation of H2S into SO2. This unexpected chemistry was explored experimentally and computationally revealing a concentration dependent branching between oxidized and elemental sulfur products. The factors affecting the final state of sulfur and the stoichiometry of the products are discussed along with their implications for H2 production, especially in a refinery setting. Hydrocarbon desulfurization is currently a major consumer of H2, but with this newly discovered chemistry, it could become a net H2 producer. This change could reduce CO2 emissions by tens of millions of tons per year.
中文翻译:

ħ 2代选自H 2氧,氢2 s至碘值周期
期间H的热化学方法调查2选自H代2 S,我们按照以下反应,H观察到意想不到的产物的形成2 S + 3I 2 + 2H 2 O→6HI + SO 2。当与公知的催化分解耦合,2HI→H 2 + I 2,这使得形成的3H 2每股H 2 S以基于反应反应,H 2 S + 2H 2 O→3H 2 + SO 2。经过重述,这两个反应共同完成了H氧化驱动的H 2 O分解2 S转化为SO 2。通过实验和计算探索了这种出乎意料的化学反应,揭示了氧化和元素硫产物之间的浓度依赖性分支。讨论了影响硫的最终状态和产品化学计量的因素,以及它们对H 2生产的影响,尤其是在炼油厂中。碳氢化合物脱硫目前是H 2的主要消耗者,但是通过这种新发现的化学方法,它有可能成为H 2的净生产者。这种变化每年可以减少数千万吨的CO 2排放量。
更新日期:2019-03-13
中文翻译:

ħ 2代选自H 2氧,氢2 s至碘值周期
期间H的热化学方法调查2选自H代2 S,我们按照以下反应,H观察到意想不到的产物的形成2 S + 3I 2 + 2H 2 O→6HI + SO 2。当与公知的催化分解耦合,2HI→H 2 + I 2,这使得形成的3H 2每股H 2 S以基于反应反应,H 2 S + 2H 2 O→3H 2 + SO 2。经过重述,这两个反应共同完成了H氧化驱动的H 2 O分解2 S转化为SO 2。通过实验和计算探索了这种出乎意料的化学反应,揭示了氧化和元素硫产物之间的浓度依赖性分支。讨论了影响硫的最终状态和产品化学计量的因素,以及它们对H 2生产的影响,尤其是在炼油厂中。碳氢化合物脱硫目前是H 2的主要消耗者,但是通过这种新发现的化学方法,它有可能成为H 2的净生产者。这种变化每年可以减少数千万吨的CO 2排放量。







































 京公网安备 11010802027423号
京公网安备 11010802027423号