当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chasing Aqueous Biphasic Systems from Simple Salts by Exploring the LiTFSI/LiCl/H2O Phase Diagram
ACS Central Science ( IF 12.7 ) Pub Date : 2019-03-12 00:00:00 , DOI: 10.1021/acscentsci.8b00955 Nicolas Dubouis 1, 2, 3 , Chanbum Park 4, 5 , Michaël Deschamps 3, 6 , Soufiane Abdelghani-Idrissi 7 , Matej Kanduč 4, 8 , Annie Colin 7 , Mathieu Salanne 3, 9 , Joachim Dzubiella 4, 10 , Alexis Grimaud 1, 2, 3 , Benjamin Rotenberg 3, 9
ACS Central Science ( IF 12.7 ) Pub Date : 2019-03-12 00:00:00 , DOI: 10.1021/acscentsci.8b00955 Nicolas Dubouis 1, 2, 3 , Chanbum Park 4, 5 , Michaël Deschamps 3, 6 , Soufiane Abdelghani-Idrissi 7 , Matej Kanduč 4, 8 , Annie Colin 7 , Mathieu Salanne 3, 9 , Joachim Dzubiella 4, 10 , Alexis Grimaud 1, 2, 3 , Benjamin Rotenberg 3, 9
Affiliation
|
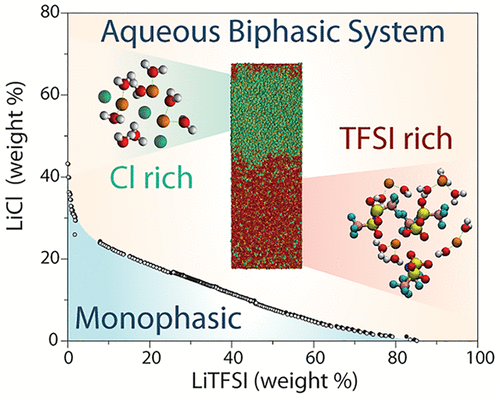
Aqueous biphasic systems (ABSs), in which two aqueous phases with different compositions coexist as separate liquids, were first reported more than a century ago with polymer solutions. Recent observations of ABS forming from concentrated mixtures of inorganic salts and ionic liquids raise the fundamental question of how “different” the components of such mixtures should be for a liquid–liquid phase separation to occur. Here we show that even two monovalent salts sharing a common cation (lithium) but with different anions, namely, LiCl and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI), may result in the formation of ABSs over a wide range of compositions at room temperature. Using a combination of experimental techniques and molecular simulations, we analyze the coexistence diagram and the mechanism driving the phase separation, arising from the different anion sizes. The understanding and control of ABS may provide new avenues for aqueous-based battery systems.
中文翻译:

通过研究LiTFSI / LiCl / H 2 O相图,从简单盐中追踪水相系统
早在一个多世纪以前,人们就报道了水性双相系统(ABS),其中两种具有不同组成的水相以独立的液体形式共存。由无机盐和离子液体的浓缩混合物形成的ABS的最新观察提出了一个基本问题,即这种混合物的成分应如何“不同”才能发生液-液相分离。在这里,我们显示,即使是两个具有相同阳离子(锂)但具有不同阴离子的一价盐,即LiCl和双(三氟甲磺酰基)酰亚胺锂(LiTFSI),也可能导致室温下在各种组成范围内形成ABS。 。结合实验技术和分子模拟,我们分析了共存图和驱动相分离的机制,由于阴离子大小不同而产生的。对ABS的理解和控制可能为水性电池系统提供新的途径。
更新日期:2019-03-12
中文翻译:

通过研究LiTFSI / LiCl / H 2 O相图,从简单盐中追踪水相系统
早在一个多世纪以前,人们就报道了水性双相系统(ABS),其中两种具有不同组成的水相以独立的液体形式共存。由无机盐和离子液体的浓缩混合物形成的ABS的最新观察提出了一个基本问题,即这种混合物的成分应如何“不同”才能发生液-液相分离。在这里,我们显示,即使是两个具有相同阳离子(锂)但具有不同阴离子的一价盐,即LiCl和双(三氟甲磺酰基)酰亚胺锂(LiTFSI),也可能导致室温下在各种组成范围内形成ABS。 。结合实验技术和分子模拟,我们分析了共存图和驱动相分离的机制,由于阴离子大小不同而产生的。对ABS的理解和控制可能为水性电池系统提供新的途径。






































 京公网安备 11010802027423号
京公网安备 11010802027423号