当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-Heterocyclic Germylene and Stannylene Catalyzed Cyanosilylation and Hydroboration of Aldehydes
Organometallics ( IF 2.5 ) Pub Date : 2019-03-11 , DOI: 10.1021/acs.organomet.8b00673
Rajarshi Dasgupta 1 , Shubhajit Das 2 , Shweta Hiwase 1 , Swapan K. Pati 2 , Shabana Khan 1
Organometallics ( IF 2.5 ) Pub Date : 2019-03-11 , DOI: 10.1021/acs.organomet.8b00673
Rajarshi Dasgupta 1 , Shubhajit Das 2 , Shweta Hiwase 1 , Swapan K. Pati 2 , Shabana Khan 1
Affiliation
|
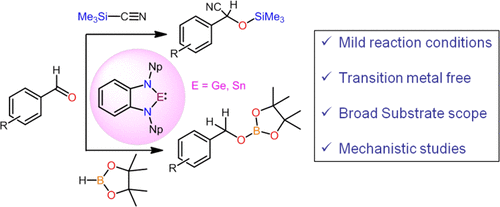
Recent years have witnessed a significant growth in the area of low-valent main-group compounds due to their potential to activate small molecules. However, there is a paucity of examples of low-valent main-group compounds being used as single-site catalysts for organic transformations. This study represents the hydroboration and cyanosilylation reactions of a range of aldehydes by a benzannulated heavier N-heterocyclic germylene (1) and stannylene (2) under mild conditions. A wide variety of substrate scope was studied. The mechanistic pathway of the cyanosilylation reaction is initiated through the coordination of TMSCN with the catalyst followed by the attack of aldehydes. Conversely, hydroboration proceeds via formation of a donor–acceptor adduct between HBpin and the catalyst. Experimental and theoretical studies were performed to establish the mechanism.
中文翻译:

N-杂环锗烯和亚锡烷基催化的氰基硅烷化和氢硼化
近年来,由于低价主族化合物具有激活小分子的潜力,因此其发展显着。但是,很少有低价主族化合物用作有机转化的单中心催化剂的例子。这项研究代表了苯甲环化的较重N-杂环亚二甲基亚砜(1)和亚苯乙烯(2)的一系列醛的硼氢化和氰基硅烷化反应)在温和的条件下。研究了各种各样的基材范围。氰基硅烷化反应的机理途径是通过TMSCN与催化剂的配位,然后是醛的进攻而引发的。相反,硼氢化通过在HBpin和催化剂之间形成供体-受体加合物而进行。进行了实验和理论研究以建立机理。
更新日期:2019-03-12
中文翻译:

N-杂环锗烯和亚锡烷基催化的氰基硅烷化和氢硼化
近年来,由于低价主族化合物具有激活小分子的潜力,因此其发展显着。但是,很少有低价主族化合物用作有机转化的单中心催化剂的例子。这项研究代表了苯甲环化的较重N-杂环亚二甲基亚砜(1)和亚苯乙烯(2)的一系列醛的硼氢化和氰基硅烷化反应)在温和的条件下。研究了各种各样的基材范围。氰基硅烷化反应的机理途径是通过TMSCN与催化剂的配位,然后是醛的进攻而引发的。相反,硼氢化通过在HBpin和催化剂之间形成供体-受体加合物而进行。进行了实验和理论研究以建立机理。

































 京公网安备 11010802027423号
京公网安备 11010802027423号