当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Addressing the Chemistry of Germacrene A by Isotope Labeling Experiments
Organic Letters ( IF 4.9 ) Pub Date : 2019-03-12 00:00:00 , DOI: 10.1021/acs.orglett.9b00725 Jan Rinkel 1 , Jeroen S. Dickschat 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-03-12 00:00:00 , DOI: 10.1021/acs.orglett.9b00725 Jan Rinkel 1 , Jeroen S. Dickschat 1
Affiliation
|
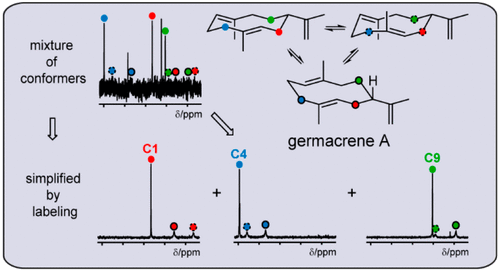
Despite the central role of germacrene A in sesquiterpene biosynthesis and its widespread occurrence in nature, its complete NMR characterization is still pending. This problem was solved through enzymatic preparation of germacrene A isotopomers that allowed for a full signal assignment to all three conformers. The obtained materials gave insights into the stereochemical course of the Cope rearrangement to β-elemene and uncovered the Cope rearrangement as a new EI-MS fragmentation reaction.
中文翻译:

通过同位素标记实验解决Germacrene A的化学反应
尽管胚ac烯A在倍半萜生物合成中起着核心作用,并且在自然界中广泛存在,但其完整的NMR表征仍在进行中。该问题通过酶法制备的ac草烯A异构体得以解决,该异构体可将信号完全分配给所有三个构象异构体。获得的材料提供了Cope重排为β-榄香烯的立体化学过程的见解,并揭示了Cope重排作为一种新的EI-MS片段化反应。
更新日期:2019-03-12
中文翻译:

通过同位素标记实验解决Germacrene A的化学反应
尽管胚ac烯A在倍半萜生物合成中起着核心作用,并且在自然界中广泛存在,但其完整的NMR表征仍在进行中。该问题通过酶法制备的ac草烯A异构体得以解决,该异构体可将信号完全分配给所有三个构象异构体。获得的材料提供了Cope重排为β-榄香烯的立体化学过程的见解,并揭示了Cope重排作为一种新的EI-MS片段化反应。































 京公网安备 11010802027423号
京公网安备 11010802027423号