Journal of Solid State Chemistry ( IF 3.2 ) Pub Date : 2019-03-11 , DOI: 10.1016/j.jssc.2019.01.036 Emily M. Reynolds , Michelle Yu , Gordon J. Thorogood , Helen E.A. Brand , Frederic Poineau , Brendan J. Kennedy
|
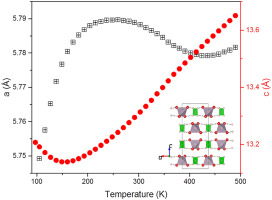
The thermal expansion of NH4TcO4 and NH4ReO4 between 97 and 500 K has been established using variable temperature synchrotron X-ray diffraction. Both oxides display a tetragonal scheelite-type structure over the temperature range. The observed anomalous thermal expansion is associated with disorder in the orientation of the NH4 groups and the presence of anisotropic, equatorial and axial, interactions between the H atoms and the BO4 tetrahedra. The importance of the hydrogen interactions was verified by a study of the isostructural compound NaReO4 over a similar temperature range. The differences in the details of the Negative Thermal Expansion (NTE) behaviour of NH4TcO4 and NH4ReO4 is a consequence of differences in the H-BO4 interactions due to changes in the B-O bond lengths within the BO4 groups. Both ammonium salts decompose when heated above ∼500 K.
中文翻译:

高tech酸铵和高r酸铵的热膨胀
NH 4 TcO 4和NH 4 ReO 4在97 K至500 K之间的热膨胀已通过使用变温同步加速器X射线衍射确定。两种氧化物在该温度范围内均显示出四方白钨矿型结构。观察到的异常热膨胀与NH 4基团的取向无序以及H原子与B O 4四面体之间存在各向异性的,赤道的和轴向的相互作用有关。氢相互作用的重要性已通过对同构化合物NaReO 4的研究得到证实。在相似的温度范围内。在NH的负热膨胀(NTE)行为的细节上的不同4 TCO 4和NH 4 REO 4是在H-差异的后果乙ö 4相互作用由于变化乙所述内-O键长乙O 4组。当加热到约500 K以上时,两种铵盐都会分解。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号