当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cis-Cisoid Helical Structures of Poly(3,5-disubstituted phenylacetylene)s Stabilized by Intramolecular n → π* Interactions
Macromolecules ( IF 5.1 ) Pub Date : 2018-02-09 00:00:00 , DOI: 10.1021/acs.macromol.8b00078 Sheng Wang 1 , Ge Shi 1 , Xiaoyan Guan 1 , Jie Zhang 1 , Xinhua Wan 1
Macromolecules ( IF 5.1 ) Pub Date : 2018-02-09 00:00:00 , DOI: 10.1021/acs.macromol.8b00078 Sheng Wang 1 , Ge Shi 1 , Xiaoyan Guan 1 , Jie Zhang 1 , Xinhua Wan 1
Affiliation
|
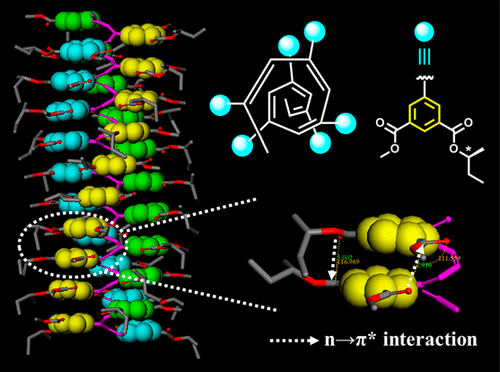
A pair of enantiomeric cis-poly(phenylacetylene)s (PPAs) substituted at the meta-positions of pendant phenyl rings by an achiral methoxycarbonyl group and a chiral 1-methylpropyloxycarbonyl group (i.e., sP-Me-C4/rP-Me-C4) as well as two cis-PPAs bearing either just a methoxycarbonyl (i.e., m-aP-Me) or a 1-methylpropyloxycarbonyl (i.e., m-sP-C4) meta substituent were synthesized under the catalysis of [Rh(nbd)Cl]2 (nbd = norbornadiene). Various techniques including 1H NMR, FTIR, Raman, UV–vis, CD, DSC, STM, DLS/SLS, and computer calculation were applied to characterize the helical structures of these polymers in both solution and solid states. sP-Me-C4/rP-Me-C4 adopted contracted cis-cisoid helical conformations in THF, toluene, CH2Cl2, and ClCH2CH2Cl but cis-transoid ones in CHCl3 and Cl2CHCHCl2. The cis-cisoid helices were considered to be stabilized by the existence of six n → π* interaction bands along the polyene backbones between vicinal carbonyl groups. Such interactions were insensitive to the dielectric constant and polarity of solvent but sensitive to the hydrogen bond donating ability of solvent and temperature. In hydrogen bond accepting solvent, the cis-cisoid helical structures were thermal stable, whereas the cis-cisoid to cis-transoid helix transition could be triggered by raising temperature in the hydrogen bond donating solvent. The stronger the hydrogen bondings between solvent molecules and carbonyl groups, the lower the temperature required to maintain cis-cisoid helix. Moreover, the cis-cisoid helices stabilized by intramolecular n → π* interactions had better tolerance to the polarity of solvent and faster recovery than those stabilized by intramolecular hydrogen bonds. No matter whether methoxycarbonyl or 1-methylpropyloxycarbonyl group was removed, only a cis-transoid helix was observed, implying the weak nature of n → π* interaction and the need for a delicate macromolecular design. This work provided an unusual strategy to build cis-cisoid PPAs.
中文翻译:

分子内n→π*相互作用稳定的聚(3,5-二取代苯基乙炔)s的顺式-Cisoid螺旋结构
一对对映体的顺式聚苯乙炔(PPA),在侧苯环的间位被一个非手性甲氧羰基和一个手性1-甲基丙氧羰基(即,sP-Me-C4 / rP-Me-C4)以及两个仅带有甲氧基羰基(即m -aP-Me)或1-甲基丙氧基羰基(即m -sP-C4)间取代基的顺式-PPA在[Rh(nbd)Cl ] 2(nbd =降冰片二烯)。各种技术,包括11 H NMR,FTIR,Raman,UV-vis,CD,DSC,STM,DLS / SLS和计算机计算被用来表征这些聚合物在溶液和固态下的螺旋结构。SP-ME-C4 / RP-ME-C4采用收缩顺式cisoid在THF,甲苯,CH螺旋构象2氯2,和CLCH 2 CH 2氯但顺式transoid在CHCl者3和Cl 2 CHCHCl 2。该顺cisoid沿邻位羰基之间的多烯主链沿六个n→π*相互作用带的存在,可以认为螺旋结构是稳定的。这种相互作用对溶剂的介电常数和极性不敏感,但是对溶剂的氢键给定能力和温度敏感。在接受氢键的溶剂中,顺式-cisoid螺旋结构是热稳定的,而在供氢键的溶剂中升高温度可以触发顺式-cisoid到顺式-transoid螺旋的转变。溶剂分子和羰基之间的氢键越强,维持顺式-cisoid螺旋所需的温度越低。而且,顺式-顺式通过分子内n→π*相互作用稳定的螺旋比通过分子内氢键稳定的螺旋具有更好的耐溶剂极性和更快的回收率。无论是否除去甲氧基羰基或1-甲基丙氧基羰基,仅观察到顺式-反式螺旋,这意味着n→π*相互作用的弱性以及对精细大分子设计的需求。这项工作提供了一个不寻常的策略来建立顺式-cisoid PPAs。
更新日期:2018-02-09
中文翻译:

分子内n→π*相互作用稳定的聚(3,5-二取代苯基乙炔)s的顺式-Cisoid螺旋结构
一对对映体的顺式聚苯乙炔(PPA),在侧苯环的间位被一个非手性甲氧羰基和一个手性1-甲基丙氧羰基(即,sP-Me-C4 / rP-Me-C4)以及两个仅带有甲氧基羰基(即m -aP-Me)或1-甲基丙氧基羰基(即m -sP-C4)间取代基的顺式-PPA在[Rh(nbd)Cl ] 2(nbd =降冰片二烯)。各种技术,包括11 H NMR,FTIR,Raman,UV-vis,CD,DSC,STM,DLS / SLS和计算机计算被用来表征这些聚合物在溶液和固态下的螺旋结构。SP-ME-C4 / RP-ME-C4采用收缩顺式cisoid在THF,甲苯,CH螺旋构象2氯2,和CLCH 2 CH 2氯但顺式transoid在CHCl者3和Cl 2 CHCHCl 2。该顺cisoid沿邻位羰基之间的多烯主链沿六个n→π*相互作用带的存在,可以认为螺旋结构是稳定的。这种相互作用对溶剂的介电常数和极性不敏感,但是对溶剂的氢键给定能力和温度敏感。在接受氢键的溶剂中,顺式-cisoid螺旋结构是热稳定的,而在供氢键的溶剂中升高温度可以触发顺式-cisoid到顺式-transoid螺旋的转变。溶剂分子和羰基之间的氢键越强,维持顺式-cisoid螺旋所需的温度越低。而且,顺式-顺式通过分子内n→π*相互作用稳定的螺旋比通过分子内氢键稳定的螺旋具有更好的耐溶剂极性和更快的回收率。无论是否除去甲氧基羰基或1-甲基丙氧基羰基,仅观察到顺式-反式螺旋,这意味着n→π*相互作用的弱性以及对精细大分子设计的需求。这项工作提供了一个不寻常的策略来建立顺式-cisoid PPAs。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号