当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
SOX12 promotes colorectal cancer cell proliferation and metastasis by regulating asparagine synthesis.
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-03-11 , DOI: 10.1038/s41419-019-1481-9 Feng Du 1 , Jie Chen 1 , Hao Liu 1 , Yanhui Cai 2 , Tianyu Cao 1 , Weili Han 1 , Xiaofang Yi 1 , Meirui Qian 1 , Dean Tian 3 , Yongzhan Nie 1 , Kaichun Wu 1 , Daiming Fan 1 , Limin Xia 1, 3
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-03-11 , DOI: 10.1038/s41419-019-1481-9 Feng Du 1 , Jie Chen 1 , Hao Liu 1 , Yanhui Cai 2 , Tianyu Cao 1 , Weili Han 1 , Xiaofang Yi 1 , Meirui Qian 1 , Dean Tian 3 , Yongzhan Nie 1 , Kaichun Wu 1 , Daiming Fan 1 , Limin Xia 1, 3
Affiliation
|
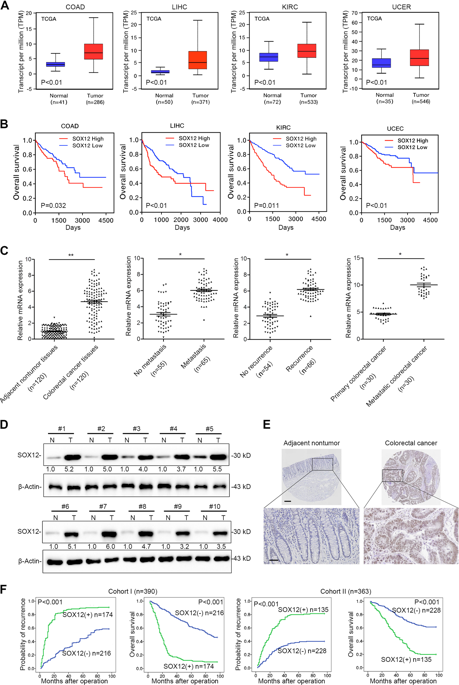
The sex-determining region Y (SRY)-box (SOX) family has a crucial role in carcinogenesis and cancer progression. However, the role of SOX12 and the mechanism by which it is dysregulated in colorectal cancer (CRC) remain unclear. Here we analyzed SOX12 expression patterns in two independent CRC cohorts (cohort I, n = 390; cohort II, n = 363) and found that SOX12 was significantly upregulated in CRC, indicating a poor prognosis in CRC patients. Overexpression of SOX12 promoted CRC cell proliferation and metastasis, whereas downregulation of SOX12 hampered CRC aggressiveness. Mechanistically, SOX12 facilitated asparagine synthesis by transactivating glutaminase (GLS), glutamic oxaloacetic transaminase 2 (GOT2), and asparagine synthetase (ASNS). Downregulation of GLS, GOT2, and ASNS blocked SOX12-mediated CRC cell proliferation and metastasis, whereas ectopic expression of GLS, GOT2, and ASNS attenuated the SOX12 knockdown-induced suppression of CRC progression. In addition, serial deletion, site-directed mutagenesis, luciferase reporter, and chromatin immunoprecipitation (ChIP) assays indicated that hypoxia-inducible factor 1α (HIF-1α) directly binds to the SOX12 promoter and induces SOX12 expression. Administration of L-asparaginase decreased SOX12-mediated tumor growth and metastasis. In human CRC samples, SOX12 expression positively correlated with GLS, GOT2, ASNS, and HIF-1α expression. Based on these results, SOX12 may serve as a prognostic biomarker and L-asparaginase represents a potential novel therapeutic agent for CRC.
中文翻译:

SOX12通过调节天冬酰胺的合成来促进结直肠癌细胞的增殖和转移。
性别决定区Y(SRY)-box(SOX)家族在致癌和癌症进展中起着至关重要的作用。但是,尚不清楚SOX12在大肠癌(CRC)中的作用及其失调的机制。在这里,我们分析了两个独立的CRC队列(队列I,n = 390;队列II,n = 363)中的SOX12表达模式,发现SOX12在CRC中显着上调,表明CRC患者的预后不良。SOX12的过表达促进CRC细胞的增殖和转移,而SOX12的下调则阻碍了CRC的侵袭性。从机制上讲,SOX12通过反式激活谷氨酰胺酶(GLS),谷氨酸草酰乙酸转氨酶2(GOT2)和天冬酰胺合成酶(ASNS)促进了天冬酰胺的合成。GLS,GOT2和ASNS的下调阻止了SOX12介导的CRC细胞增殖和转移,而GLS,GOT2和ASNS的异位表达减弱了SOX12敲低诱导的CRC进展的抑制作用。此外,连续缺失,定点诱变,荧光素酶报告基因和染色质免疫沉淀(ChIP)分析表明,缺氧诱导因子1α(HIF-1α)直接与SOX12启动子结合并诱导SOX12表达。L-天冬酰胺酶的施用减少了SOX12介导的肿瘤生长和转移。在人类CRC样本中,SOX12表达与GLS,GOT2,ASNS和HIF-1α表达正相关。基于这些结果,SOX12可以作为预后的生物标志物,而L-天冬酰胺酶则可以作为CRC的潜在新型治疗剂。定点诱变,荧光素酶报告基因和染色质免疫沉淀(ChIP)分析表明,缺氧诱导因子1α(HIF-1α)直接与SOX12启动子结合并诱导SOX12表达。L-天冬酰胺酶的施用减少了SOX12介导的肿瘤生长和转移。在人类CRC样本中,SOX12表达与GLS,GOT2,ASNS和HIF-1α表达正相关。基于这些结果,SOX12可以作为预后的生物标志物,而L-天冬酰胺酶则可以作为CRC的潜在新型治疗剂。定点诱变,荧光素酶报告基因和染色质免疫沉淀(ChIP)分析表明,缺氧诱导因子1α(HIF-1α)直接与SOX12启动子结合并诱导SOX12表达。L-天冬酰胺酶的施用减少了SOX12介导的肿瘤生长和转移。在人类CRC样本中,SOX12表达与GLS,GOT2,ASNS和HIF-1α表达正相关。基于这些结果,SOX12可以作为预后的生物标志物,而L-天冬酰胺酶则可以作为CRC的潜在新型治疗剂。SOX12表达与GLS,GOT2,ASNS和HIF-1α表达呈正相关。基于这些结果,SOX12可以作为预后的生物标志物,而L-天冬酰胺酶则可以作为CRC的潜在新型治疗剂。SOX12表达与GLS,GOT2,ASNS和HIF-1α表达呈正相关。基于这些结果,SOX12可以作为预后的生物标志物,而L-天冬酰胺酶则可以作为CRC的潜在新型治疗剂。
更新日期:2019-03-11
中文翻译:

SOX12通过调节天冬酰胺的合成来促进结直肠癌细胞的增殖和转移。
性别决定区Y(SRY)-box(SOX)家族在致癌和癌症进展中起着至关重要的作用。但是,尚不清楚SOX12在大肠癌(CRC)中的作用及其失调的机制。在这里,我们分析了两个独立的CRC队列(队列I,n = 390;队列II,n = 363)中的SOX12表达模式,发现SOX12在CRC中显着上调,表明CRC患者的预后不良。SOX12的过表达促进CRC细胞的增殖和转移,而SOX12的下调则阻碍了CRC的侵袭性。从机制上讲,SOX12通过反式激活谷氨酰胺酶(GLS),谷氨酸草酰乙酸转氨酶2(GOT2)和天冬酰胺合成酶(ASNS)促进了天冬酰胺的合成。GLS,GOT2和ASNS的下调阻止了SOX12介导的CRC细胞增殖和转移,而GLS,GOT2和ASNS的异位表达减弱了SOX12敲低诱导的CRC进展的抑制作用。此外,连续缺失,定点诱变,荧光素酶报告基因和染色质免疫沉淀(ChIP)分析表明,缺氧诱导因子1α(HIF-1α)直接与SOX12启动子结合并诱导SOX12表达。L-天冬酰胺酶的施用减少了SOX12介导的肿瘤生长和转移。在人类CRC样本中,SOX12表达与GLS,GOT2,ASNS和HIF-1α表达正相关。基于这些结果,SOX12可以作为预后的生物标志物,而L-天冬酰胺酶则可以作为CRC的潜在新型治疗剂。定点诱变,荧光素酶报告基因和染色质免疫沉淀(ChIP)分析表明,缺氧诱导因子1α(HIF-1α)直接与SOX12启动子结合并诱导SOX12表达。L-天冬酰胺酶的施用减少了SOX12介导的肿瘤生长和转移。在人类CRC样本中,SOX12表达与GLS,GOT2,ASNS和HIF-1α表达正相关。基于这些结果,SOX12可以作为预后的生物标志物,而L-天冬酰胺酶则可以作为CRC的潜在新型治疗剂。定点诱变,荧光素酶报告基因和染色质免疫沉淀(ChIP)分析表明,缺氧诱导因子1α(HIF-1α)直接与SOX12启动子结合并诱导SOX12表达。L-天冬酰胺酶的施用减少了SOX12介导的肿瘤生长和转移。在人类CRC样本中,SOX12表达与GLS,GOT2,ASNS和HIF-1α表达正相关。基于这些结果,SOX12可以作为预后的生物标志物,而L-天冬酰胺酶则可以作为CRC的潜在新型治疗剂。SOX12表达与GLS,GOT2,ASNS和HIF-1α表达呈正相关。基于这些结果,SOX12可以作为预后的生物标志物,而L-天冬酰胺酶则可以作为CRC的潜在新型治疗剂。SOX12表达与GLS,GOT2,ASNS和HIF-1α表达呈正相关。基于这些结果,SOX12可以作为预后的生物标志物,而L-天冬酰胺酶则可以作为CRC的潜在新型治疗剂。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号