当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Migratory Reductive Acylation between Alkyl Halides or Alkenes and Alkyl Carboxylic Acids by Nickel Catalysis
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-03-11 00:00:00 , DOI: 10.1021/acscatal.9b00521
Jun He 1 , Peihong Song 1 , Xianfeng Xu 1 , Shaolin Zhu 1 , You Wang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-03-11 00:00:00 , DOI: 10.1021/acscatal.9b00521
Jun He 1 , Peihong Song 1 , Xianfeng Xu 1 , Shaolin Zhu 1 , You Wang 1
Affiliation
|
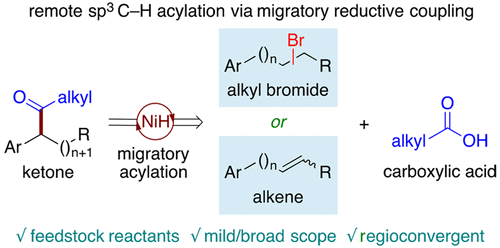
A mild migratory reductive acyl cross-coupling has been achieved through NiH-catalyzed chainwalking and subsequent cross-coupling from two abundant starting materials, alkyl bromides, and carboxylic acids. This strategy allows the direct acylation of the benzylic sp3 C–H bond with high yield as a single regioisomer. As an alternative, the alkyl bromide could be replaced by the proposed olefin intermediate and commercially available n-PrBr to achieve a remote hydroacylation process.
中文翻译:

镍催化的卤代烷或烯烃与烷基羧酸之间的迁移还原酰化
通过NiH催化的链走反应以及随后从两种丰富的起始原料(烷基溴化物和羧酸)的交叉偶联,实现了温和的迁移性还原性酰基交叉偶联。这种策略可以将苄基sp 3 C–H键直接酰化,并以高收率作为单一的区域异构体。作为替代,烷基溴可以用提出的烯烃中间体和可商购的正-PrBr代替,以实现远程加氢酰化过程。
更新日期:2019-03-11
中文翻译:

镍催化的卤代烷或烯烃与烷基羧酸之间的迁移还原酰化
通过NiH催化的链走反应以及随后从两种丰富的起始原料(烷基溴化物和羧酸)的交叉偶联,实现了温和的迁移性还原性酰基交叉偶联。这种策略可以将苄基sp 3 C–H键直接酰化,并以高收率作为单一的区域异构体。作为替代,烷基溴可以用提出的烯烃中间体和可商购的正-PrBr代替,以实现远程加氢酰化过程。

































 京公网安备 11010802027423号
京公网安备 11010802027423号