当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Low-dose oral copper treatment changes the hippocampal phosphoproteomic profile and perturbs mitochondrial function in a mouse model of Alzheimer's disease.
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2019-03-09 , DOI: 10.1016/j.freeradbiomed.2019.03.002 Chongyang Chen 1 , Xin Jiang 2 , Yingchao Li 1 , Haitao Yu 3 , Shupeng Li 4 , Zaijun Zhang 5 , Hua Xu 6 , Ying Yang 7 , Gongping Liu 7 , Feiqi Zhu 8 , Xiaohu Ren 3 , Liangyu Zou 9 , Benhong Xu 3 , Jianjun Liu 3 , Peter S Spencer 10 , Xifei Yang 3
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2019-03-09 , DOI: 10.1016/j.freeradbiomed.2019.03.002 Chongyang Chen 1 , Xin Jiang 2 , Yingchao Li 1 , Haitao Yu 3 , Shupeng Li 4 , Zaijun Zhang 5 , Hua Xu 6 , Ying Yang 7 , Gongping Liu 7 , Feiqi Zhu 8 , Xiaohu Ren 3 , Liangyu Zou 9 , Benhong Xu 3 , Jianjun Liu 3 , Peter S Spencer 10 , Xifei Yang 3
Affiliation
|
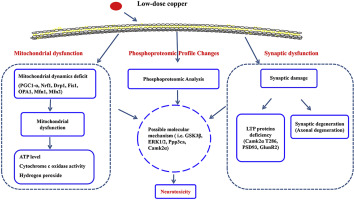
Excessive copper can cause neurotoxicity and contribute to the development of some neurological diseases; however, copper neurotoxicity and the potential mechanisms remain poorly understood. We used proteomics and phosphoproteomics to quantify protein changes in the hippocampus of wild-type and 3xTg-AD mice, both of which were treated at 6 months of age with 2 months of drinking water with or without added copper chloride (0.13 ppm concentration). A total of 3960 unique phosphopeptides (5290 phosphorylation sites) from 1406 phosphoproteins was identified. Differentially expressed phosphoproteins involved neuronal and synaptic function, transcriptional regulation, energy metabolism and mitochondrial function. In addition, low-dose copper treatment of wild-type mice decreased hippocampal mitochondrial copy number, mitochondrial biogenesis and disrupted mitochondrial dynamics; these changes were associated with increased hydrogen peroxide production (H2O2), reduced cytochrome oxidase activity and decreased ATP content. In 3xTg-AD mice, identical low-dose oral copper treatment increased axonal degeneration, which was associated with altered phosphorylation of Camk2α at T286 and phosphorylation of mitogen-activated protein kinase (ERK1/2), which involved long-term potentiation (LTP) signaling. Mitochondrial dysfunction was mainly related to changes in phosphorylation levels of glycogen synthase kinase-3 beta (GSK3β) and serine/threonine-protein phosphatase 2B catalytic subunit alpha isoform (Ppp3ca), which involved mitochondrial biogenesis signaling. In sum, low-dose oral copper treatment changes the phosphorylation of key hippocampal proteins involved in mitochondrial, synaptic and axonal integrity. These data showing that excess of copper speeds some early events of AD changes observed suggest that excess circulating copper has the potential to perturb brain function of wild-type mice and exacerbate neurodegenerative changes in a mouse model of AD.
中文翻译:

在阿尔茨海默氏病小鼠模型中,低剂量口服铜治疗可改变海马磷酸化蛋白质组学特征并扰乱线粒体功能。
过量的铜会引起神经毒性,并导致某些神经系统疾病的发展。然而,对铜的神经毒性及其潜在机制仍知之甚少。我们使用蛋白质组学和磷酸化蛋白质组学来量化野生型和3xTg-AD小鼠海马中的蛋白质变化,这两种小鼠均在6个月大时接受2个月饮用水处理,添加或不添加氯化铜(0.13 ppm浓度)。从1406个磷蛋白中鉴定出总共3960个独特的磷酸肽(5290个磷酸化位点)。差异表达的磷蛋白涉及神经元和突触功能,转录调节,能量代谢和线粒体功能。此外,低剂量铜治疗野生型小鼠可降低海马线粒体拷贝数,线粒体的生物发生和线粒体动力学的破坏;这些变化与过氧化氢产量(H2O2)的增加,细胞色素氧化酶活性的降低和ATP含量的降低有关。在3xTg-AD小鼠中,相同的低剂量口服铜治疗会增加轴突变性,这与T286处Camk2α的磷酸化改变和丝裂原激活的蛋白激酶(ERK1 / 2)的磷酸化有关,这涉及长期增强(LTP)信号。线粒体功能障碍主要与糖原合酶激酶3β(GSK3β)和丝氨酸/苏氨酸蛋白磷酸酶2B催化亚基α亚型(Ppp3ca)的磷酸化水平变化有关,涉及线粒体生物发生信号。总共,低剂量口服铜治疗可改变涉及线粒体,突触和轴突完整性的关键海马蛋白的磷酸化。这些数据表明过量的铜会加速某些AD变化的早期事件,这表明过量的循环铜有可能扰动野生型小鼠的脑功能并加剧AD模型的神经退行性变化。
更新日期:2019-03-09
中文翻译:

在阿尔茨海默氏病小鼠模型中,低剂量口服铜治疗可改变海马磷酸化蛋白质组学特征并扰乱线粒体功能。
过量的铜会引起神经毒性,并导致某些神经系统疾病的发展。然而,对铜的神经毒性及其潜在机制仍知之甚少。我们使用蛋白质组学和磷酸化蛋白质组学来量化野生型和3xTg-AD小鼠海马中的蛋白质变化,这两种小鼠均在6个月大时接受2个月饮用水处理,添加或不添加氯化铜(0.13 ppm浓度)。从1406个磷蛋白中鉴定出总共3960个独特的磷酸肽(5290个磷酸化位点)。差异表达的磷蛋白涉及神经元和突触功能,转录调节,能量代谢和线粒体功能。此外,低剂量铜治疗野生型小鼠可降低海马线粒体拷贝数,线粒体的生物发生和线粒体动力学的破坏;这些变化与过氧化氢产量(H2O2)的增加,细胞色素氧化酶活性的降低和ATP含量的降低有关。在3xTg-AD小鼠中,相同的低剂量口服铜治疗会增加轴突变性,这与T286处Camk2α的磷酸化改变和丝裂原激活的蛋白激酶(ERK1 / 2)的磷酸化有关,这涉及长期增强(LTP)信号。线粒体功能障碍主要与糖原合酶激酶3β(GSK3β)和丝氨酸/苏氨酸蛋白磷酸酶2B催化亚基α亚型(Ppp3ca)的磷酸化水平变化有关,涉及线粒体生物发生信号。总共,低剂量口服铜治疗可改变涉及线粒体,突触和轴突完整性的关键海马蛋白的磷酸化。这些数据表明过量的铜会加速某些AD变化的早期事件,这表明过量的循环铜有可能扰动野生型小鼠的脑功能并加剧AD模型的神经退行性变化。













































 京公网安备 11010802027423号
京公网安备 11010802027423号