当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Site-Specific 1D and 2D IR Spectroscopy to Characterize the Conformations and Dynamics of Protein Molecular Recognition.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2019-03-21 , DOI: 10.1021/acs.jpcb.9b00969
Sashary Ramos 1 , Megan C Thielges 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2019-03-21 , DOI: 10.1021/acs.jpcb.9b00969
Sashary Ramos 1 , Megan C Thielges 1
Affiliation
|
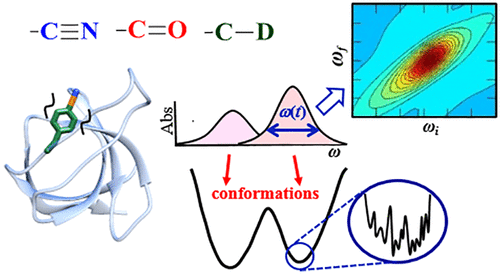
Proteins exist as ensembles of interconverting states on a complex energy landscape. A complete, molecular-level understanding of their function requires knowledge of the populated states and thus the experimental tools to characterize them. Infrared (IR) spectroscopy has an inherently fast time scale that can capture all states and their dynamics with, in principle, bond-specific spatial resolution, and 2D IR methods that provide richer information are becoming more routine. Although application of IR spectroscopy for investigation of proteins is challenged by spectral congestion, the issue can be overcome by site-specific introduction of amino acid side chains that have IR probe groups with frequency-resolved absorptions, which furthermore enables selective characterization of different locations in proteins. Here, we briefly introduce the biophysical methods and summarize the current progress toward the study of proteins. We then describe our efforts to apply site-specific 1D and 2D IR spectroscopy toward elucidation of protein conformations and dynamics to investigate their involvement in protein molecular recognition, in particular mediated by dynamic complexes: plastocyanin and its binding partner cytochrome f, cytochrome P450s and substrates or redox partners, and Src homology 3 domains and proline-rich peptide motifs. We highlight the advantages of frequency-resolved probes to characterize specific, local sites in proteins and uncover variation among different locations, as well as the advantage of the fast time scale of IR spectroscopy to detect rapidly interconverting states. In addition, we illustrate the greater insight provided by 2D methods and discuss potential routes for further advancement of the field of biomolecular 2D IR spectroscopy.
中文翻译:

特定于位点的1D和2D红外光谱用于表征蛋白质分子识别的构象和动力学。
蛋白质以复杂状态下的相互转换状态的集合形式存在。要对其分子功能有完整的分子水平的了解,就需要了解其所处的状态,因此需要用实验工具来表征它们。红外(IR)光谱具有固有的快速时标,可以通过原则上特定于键的空间分辨率捕获所有状态及其动力学,而提供更丰富信息的2D红外方法正变得越来越常规。尽管红外光谱技术在蛋白质研究中的应用受到光谱拥挤的挑战,但可以通过在位点引入具有频率分辨吸收的红外探针基团的氨基酸侧链来解决该问题,从而进一步选择性表征蛋白质中的不同位置。蛋白质。这里,我们简要介绍了生物物理方法,并总结了蛋白质研究的最新进展。然后,我们描述我们将特定于位置的1D和2D IR光谱应用于阐明蛋白质构象和动力学的努力,以研究它们在蛋白质分子识别中的参与,特别是由动态复合物介导的:质体蓝素及其结合伙伴细胞色素f,细胞色素P450和底物或氧化还原伙伴,以及Src同源性3结构域和富含脯氨酸的肽基序。我们着重介绍了频率分辨探针可表征蛋白质中特定的局部位点并揭示不同位置之间的差异的优势,以及IR光谱快速时间尺度检测快速相互转换状态的优势。此外,
更新日期:2019-03-21
中文翻译:

特定于位点的1D和2D红外光谱用于表征蛋白质分子识别的构象和动力学。
蛋白质以复杂状态下的相互转换状态的集合形式存在。要对其分子功能有完整的分子水平的了解,就需要了解其所处的状态,因此需要用实验工具来表征它们。红外(IR)光谱具有固有的快速时标,可以通过原则上特定于键的空间分辨率捕获所有状态及其动力学,而提供更丰富信息的2D红外方法正变得越来越常规。尽管红外光谱技术在蛋白质研究中的应用受到光谱拥挤的挑战,但可以通过在位点引入具有频率分辨吸收的红外探针基团的氨基酸侧链来解决该问题,从而进一步选择性表征蛋白质中的不同位置。蛋白质。这里,我们简要介绍了生物物理方法,并总结了蛋白质研究的最新进展。然后,我们描述我们将特定于位置的1D和2D IR光谱应用于阐明蛋白质构象和动力学的努力,以研究它们在蛋白质分子识别中的参与,特别是由动态复合物介导的:质体蓝素及其结合伙伴细胞色素f,细胞色素P450和底物或氧化还原伙伴,以及Src同源性3结构域和富含脯氨酸的肽基序。我们着重介绍了频率分辨探针可表征蛋白质中特定的局部位点并揭示不同位置之间的差异的优势,以及IR光谱快速时间尺度检测快速相互转换状态的优势。此外,































 京公网安备 11010802027423号
京公网安备 11010802027423号