当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reactivity of 4-Aminopyridine with Halogens and Interhalogens: Weak Interactions Supported Networks of 4-Aminopyridine and 4-Aminopyridinium
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2019-03-08 00:00:00 , DOI: 10.1021/acs.cgd.9b00119 Esa Kukkonen 1 , Henri Malinen 1 , Matti Haukka 1 , Jari Konu 1
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2019-03-08 00:00:00 , DOI: 10.1021/acs.cgd.9b00119 Esa Kukkonen 1 , Henri Malinen 1 , Matti Haukka 1 , Jari Konu 1
Affiliation
|
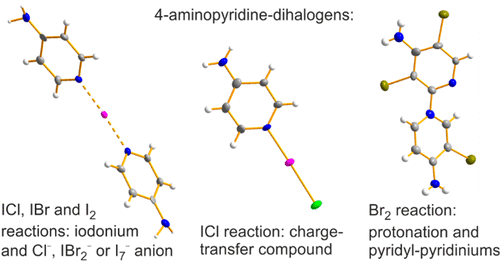
The reaction of 4-aminopyridine (4-AP) with ICl in a 1:1 molar ratio in CH2Cl2 produced the expected charge-transfer complex [4-NH2-1λ4-C5H4N-1-ICl] (1·ICl) and the ionic species [(4-NH2-1λ4-C5H4N)2-1μ-I+][Cl–] (2·Cl–) in a 2:1 relation, as indicated by 1H NMR spectroscopy in solution. In contrast, only the ionic compound [(4-NH2-1λ4-C5H4N)2-1μ-I+][IBr2–] (2·IBr2–) was observed in the analogous reaction with IBr. The reaction between 4-AP and I2 in a 1:1 molar ratio also afforded two components, one of which was identified as the congeneric cation in [(4-NH2-1λ4-C5H4N)2-1μ-I+][I7–] (2·I7–) that contains a polyiodide anion as a result of transformation in a 1:2 molar ratio between the starting materials. In all of these ionic products, the crystal structures feature an iodonium ion, I+, trapped between two 4-AP rings through N···I+···N contact. Surprisingly, the reaction of 4-AP with Br2 in CH2Cl2 resulted in an immediate protonation of the 4-aminopyridine (1H NMR) and [4-NH2-1λ4-C5H4N-1-H+][Br–] (3·Br–) was characterized as the main product. A subsequent peculiar bromination–dimerization process afforded the novel pyridyl-pyridinium cations {3,3′,5′-Br3-1λ4-[1,2′-(C5H4N)2]-4,4′-(NH2)2}+[X–] (4·Br–, 4·Br3–) and {3′,5′-Br2-1λ4-[1,2′-(C5H4N)2]-4,4′-(NH2)2}+[X–] (5·Br–, 5·Br3–). Compounds 1–5 as well as two protonated species, [4-NH2-1λ4-C5H4N-1-H+]2[Cl–][I3–] (32·Cl–·I3–) and [(4-NH2-1λ4-C5H4N)2-1μ-H+][I–] (6·I–), all display extended 3D networks supported by halogen and hydrogen bonding in the solid state.
中文翻译:

4-氨基吡啶与卤素和卤素间的反应性:弱相互作用支持4-氨基吡啶和4-氨基吡啶鎓网络
4-氨基吡啶(4-AP)与ICl的的在1中的反应:在CH 1的摩尔比2氯2产生预期的电荷转移络合物[4-NH 2 -1 λ 4 -C 5 ħ 4 N-1- ICl的](1 ·ICL)和离子物种[(4- NH 2 -1 λ 4 -C 5 ħ 4 N)2 -1μ-I + ] [氯- (2 ·氯- )以2:1如溶液中的1 H NMR光谱所示。相比之下,只有离子性化合物[(4- NH 2 -1 λ4 -C 5 ħ 4 N)2 -1μ-I + ] [IBR 2 - ](2 ·IBR 2 - )是在与IBR的类似反应观察到的。4-AP和我之间的反应2中以1:1的摩尔比也得到两个组分,其中之一被确定为[(4- NH的同类阳离子2 -1 λ 4 -C 5 ħ 4 N)2 - 1μ-I + ] [I 7 – ](2 ·I 7 –),由于原料之间以1:2的摩尔比转化而含有聚碘阴离子。在所有这些离子产物中,晶体结构都具有一个碘鎓离子I +,通过N··I + ···N接触被困在两个4-AP环之间。令人惊讶地,4-AP与溴反应2在CH 2氯2导致的4-氨基吡啶的直接质子化(1 1 H NMR)和[4-NH 2 -1 λ 4 -C 5 ħ 4 N-1- H + ] [Br – ](3 ·Br –)被定为主要产品。随后奇特溴化二聚化过程,得到新颖的吡啶基-吡啶鎓阳离子{3,3',5'-溴3 -1 λ 4 - [1,2' - (C 5 H ^ 4 N)2 ] -4,4' - (NH 2)2 } + [X - ](4 ·溴-,4 ·溴3 -)和{3',5'-溴2 -1 λ 4 - [1,2' - (C 5 H ^ 4 N)2 ] -4,4'-(NH 2)2 } + [X– ](5 ·Br –,5 ·Br 3 –)。化合物1 - 5以及两个质子化物种,[4-NH 2 -1 λ 4 -C 5 ħ 4 N-1-H + ] 2 [氯- ] [I 3 - ](3 2 ·氯- ·I 3 -)和[(4-NH 2 -1 λ 4 -C 5 ħ 4 N)2 -1μ-H + ] [I– ](6 ·I –),所有显示的3D扩展网络均受固态卤素和氢键的支持。
更新日期:2019-03-08
中文翻译:

4-氨基吡啶与卤素和卤素间的反应性:弱相互作用支持4-氨基吡啶和4-氨基吡啶鎓网络
4-氨基吡啶(4-AP)与ICl的的在1中的反应:在CH 1的摩尔比2氯2产生预期的电荷转移络合物[4-NH 2 -1 λ 4 -C 5 ħ 4 N-1- ICl的](1 ·ICL)和离子物种[(4- NH 2 -1 λ 4 -C 5 ħ 4 N)2 -1μ-I + ] [氯- (2 ·氯- )以2:1如溶液中的1 H NMR光谱所示。相比之下,只有离子性化合物[(4- NH 2 -1 λ4 -C 5 ħ 4 N)2 -1μ-I + ] [IBR 2 - ](2 ·IBR 2 - )是在与IBR的类似反应观察到的。4-AP和我之间的反应2中以1:1的摩尔比也得到两个组分,其中之一被确定为[(4- NH的同类阳离子2 -1 λ 4 -C 5 ħ 4 N)2 - 1μ-I + ] [I 7 – ](2 ·I 7 –),由于原料之间以1:2的摩尔比转化而含有聚碘阴离子。在所有这些离子产物中,晶体结构都具有一个碘鎓离子I +,通过N··I + ···N接触被困在两个4-AP环之间。令人惊讶地,4-AP与溴反应2在CH 2氯2导致的4-氨基吡啶的直接质子化(1 1 H NMR)和[4-NH 2 -1 λ 4 -C 5 ħ 4 N-1- H + ] [Br – ](3 ·Br –)被定为主要产品。随后奇特溴化二聚化过程,得到新颖的吡啶基-吡啶鎓阳离子{3,3',5'-溴3 -1 λ 4 - [1,2' - (C 5 H ^ 4 N)2 ] -4,4' - (NH 2)2 } + [X - ](4 ·溴-,4 ·溴3 -)和{3',5'-溴2 -1 λ 4 - [1,2' - (C 5 H ^ 4 N)2 ] -4,4'-(NH 2)2 } + [X– ](5 ·Br –,5 ·Br 3 –)。化合物1 - 5以及两个质子化物种,[4-NH 2 -1 λ 4 -C 5 ħ 4 N-1-H + ] 2 [氯- ] [I 3 - ](3 2 ·氯- ·I 3 -)和[(4-NH 2 -1 λ 4 -C 5 ħ 4 N)2 -1μ-H + ] [I– ](6 ·I –),所有显示的3D扩展网络均受固态卤素和氢键的支持。












































 京公网安备 11010802027423号
京公网安备 11010802027423号