Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitrosobenzene: Reagent for the Mitsunobu Esterification Reaction.
ACS Omega ( IF 3.7 ) Pub Date : 2019-03-07 , DOI: 10.1021/acsomega.8b03551 Adam Pokluda 1 , Michal Kohout 1 , Josef Chudoba 1 , Martin Krupička 1 , Radek Cibulka 1
ACS Omega ( IF 3.7 ) Pub Date : 2019-03-07 , DOI: 10.1021/acsomega.8b03551 Adam Pokluda 1 , Michal Kohout 1 , Josef Chudoba 1 , Martin Krupička 1 , Radek Cibulka 1
Affiliation
|
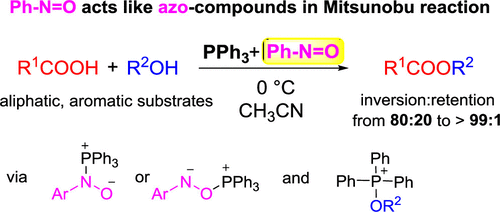
Nitrosobenzene has been demonstrated to participate in the Mitsunobu reaction in an analogous manner to dialkyl azodicarboxylates. The protocol using nitrosobenzene and triphenylphosphine (1:1) under mild conditions (0 °C) provides the ester derivatives of aliphatic and aromatic acids using various alcohols in moderate yield and with good enantioselectivity, giving the desired products predominantly with an inversion of configuration. The proposed mechanism, which is analogous to that observed using dialkyl azodicarboxylates, involves a nitrosobenzene-triphenylphosphine adduct and an alkoxytriphenylphosphonium ion and was supported by density functional theory calculations, 31P NMR spectroscopy, and experiments conducted with isotopically labeled substrates.
中文翻译:

亚硝基苯:Mitsunobu酯化反应的试剂。
已证明亚硝基苯以类似于偶氮二羧酸二烷基酯的方式参与Mitsunobu反应。在温和的条件下(0°C)使用亚硝基苯和三苯膦(1:1)的方案可使用各种醇以中等收率和良好的对映选择性提供脂族和芳族酸的酯衍生物,从而主要提供所需产物,且构型反转。所提出的机制与使用偶氮二羧酸二烷基酯所观察到的机制相似,涉及亚硝基苯-三苯基膦加合物和烷氧基三苯基ion离子,并受到密度泛函理论计算,31P NMR光谱和用同位素标记的底物进行的实验的支持。
更新日期:2019-03-07
中文翻译:

亚硝基苯:Mitsunobu酯化反应的试剂。
已证明亚硝基苯以类似于偶氮二羧酸二烷基酯的方式参与Mitsunobu反应。在温和的条件下(0°C)使用亚硝基苯和三苯膦(1:1)的方案可使用各种醇以中等收率和良好的对映选择性提供脂族和芳族酸的酯衍生物,从而主要提供所需产物,且构型反转。所提出的机制与使用偶氮二羧酸二烷基酯所观察到的机制相似,涉及亚硝基苯-三苯基膦加合物和烷氧基三苯基ion离子,并受到密度泛函理论计算,31P NMR光谱和用同位素标记的底物进行的实验的支持。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号