当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interplay between Solubility Limit, Structure, and Optical Properties of Tungsten-Doped Ga2O3 Compounds Synthesized by a Two-Step Calcination Process
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-03-07 00:00:00 , DOI: 10.1021/acs.inorgchem.8b03328 Vishal Zade 1 , Bandi Mallesham 1 , Sanjay Shantha-Kumar 2 , Arturo Bronson 2 , C. V. Ramana 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-03-07 00:00:00 , DOI: 10.1021/acs.inorgchem.8b03328 Vishal Zade 1 , Bandi Mallesham 1 , Sanjay Shantha-Kumar 2 , Arturo Bronson 2 , C. V. Ramana 1
Affiliation
|
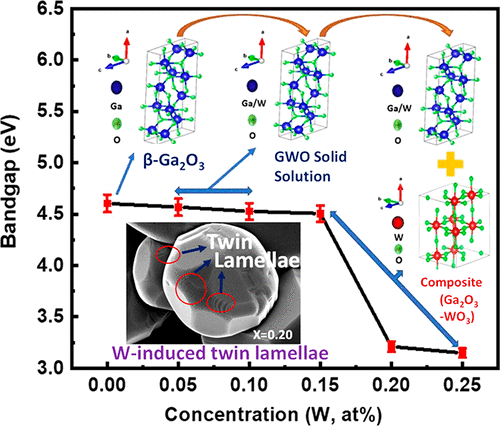
This work unfolds the fundamental mechanisms and demonstrates the tunable optical properties derived via chemical composition tailoring in tungsten (W)-doped gallium oxide (Ga2O3) compounds. On the basis of the detailed investigation, the solubility limits of tungsten (W6+) ion and associated effects on the crystal structure, morphology, and optical properties of W-doped Ga2O3 (Ga2–2xWxO3, 0.00 ≤ x ≤ 0.25, GWO) compounds are reported. GWO materials were synthesized via a conventional solid-state reaction route, where a two-step calcination is adopted to produce materials with a high structural and chemical quality. X-ray diffraction analyses of sintered GWO compounds reveal the formation of a solid solution of GWO compounds at lower concentrations W (x ≤ 0.10), while unreacted WO3 secondary phase formation occurs at higher concentrations (x>0.10). Insolubility of W at higher concentrations (x ≥ 0.15) is attributed to the difference in formation enthalpies of respective oxides, i.e., Ga2O3 and WO3. GWO compounds exhibit an interesting trend in morphology evolution as a function of W content. While intrinsic Ga2O3 exhibits rod-shaped morphology, W-doped Ga2O3 compounds exhibit nearly spherical-shaped grain morphology. Increasing W content (x ≥ 0.10) induces morphology transformation from spherical to faceted grains with different facets (square and hexagonal). Relatively larger grain sizes in GWO compounds might be attributed to vacancy assisted enhanced mass transport due to W incorporation and/or WO3 induced liquid phase sintering. Our findings demonstrate a substantial red shift in band gap (Eg), which is evident from the optical absorption spectra, enabling the wide spectral selectivity of GWO compounds. W 5d orbitals induced sp–d exchange interaction between valence band and conduction band electrons accounts for the substantial red shift in Eg of GWO compounds. Also, with increasing W, Eg decreases linearly, obeying Vegard law up to x = 0.15 and, at this point, an abrupt Eg drop prevails. The nonlinearity (bowing effect) behavior in Eg beyond x = 0.15 is due to insolubility of W at higher concentrations. The fundamental scientific understanding of the interdependence of synthetic conditions, structure, chemistry, and band gap could be useful to optimize GWO materials for optical, optoelectronic, and photocatalytic device applications.
中文翻译:

两步煅烧过程合成的掺杂钨的Ga 2 O 3化合物的溶解度极限,结构和光学性质之间的相互作用
这项工作揭示了基本机理,并证明了在掺钨(W)的氧化镓(Ga 2 O 3)化合物中通过化学成分调整获得的可调光学特性。在详细研究的基础上,钨(W 6+)离子的溶解度极限以及对掺W的Ga 2 O 3(Ga 2–2 x W x O 3)的晶体结构,形态和光学性质的相关影响,0.00≤ X报告了≤0.25,GWO)的化合物。GWO材料是通过常规的固态反应路线合成的,其中通过两步煅烧来生产具有高结构和化学质量的材料。烧结GWO化合物的X射线衍射分析表明GWO化合物的固溶体的形成在低浓度W(X ≤0.10),而未反应的WO 3的二次相的形成发生在较高浓度(X> 0.10)。在较高浓度(W中的不溶性X ≥0.15)归因于各自的氧化物,即形成焓差,镓2 ö 3和WO 3。GWO化合物在形态演变中表现出有趣的趋势,是W含量的函数。固有的Ga 2 O 3表现出棒状形态,而W掺杂的Ga 2 O 3化合物表现出近球形的晶粒形态。增加W量(X ≥0.10)诱导从球形到与不同面(方形和六角形)面的晶粒形态的转变。GWO化合物中相对较大的晶粒尺寸可能归因于W的掺入和/或WO 3诱导的液相烧结,空位辅助了增强的质量传递。我们的发现表明,带隙发生了显着的红移(E g从光吸收光谱可以明显看出),可以实现GWO化合物的宽光谱选择性。W 5d轨道在价带和导带电子之间引起sp – d交换相互作用,这解释了GWO化合物E g的大量红移。同样,随着W的增加,E g线性降低,服从Vegard定律直到x = 0.15,此时,E g突然下降。E g超过x的非线性(弯曲效应)行为= 0.15是由于W在较高浓度下不溶。对合成条件,结构,化学和带隙的相互依赖关系的基本科学理解可能有助于优化用于光学,光电和光催化装置应用的GWO材料。
更新日期:2019-03-07
中文翻译:

两步煅烧过程合成的掺杂钨的Ga 2 O 3化合物的溶解度极限,结构和光学性质之间的相互作用
这项工作揭示了基本机理,并证明了在掺钨(W)的氧化镓(Ga 2 O 3)化合物中通过化学成分调整获得的可调光学特性。在详细研究的基础上,钨(W 6+)离子的溶解度极限以及对掺W的Ga 2 O 3(Ga 2–2 x W x O 3)的晶体结构,形态和光学性质的相关影响,0.00≤ X报告了≤0.25,GWO)的化合物。GWO材料是通过常规的固态反应路线合成的,其中通过两步煅烧来生产具有高结构和化学质量的材料。烧结GWO化合物的X射线衍射分析表明GWO化合物的固溶体的形成在低浓度W(X ≤0.10),而未反应的WO 3的二次相的形成发生在较高浓度(X> 0.10)。在较高浓度(W中的不溶性X ≥0.15)归因于各自的氧化物,即形成焓差,镓2 ö 3和WO 3。GWO化合物在形态演变中表现出有趣的趋势,是W含量的函数。固有的Ga 2 O 3表现出棒状形态,而W掺杂的Ga 2 O 3化合物表现出近球形的晶粒形态。增加W量(X ≥0.10)诱导从球形到与不同面(方形和六角形)面的晶粒形态的转变。GWO化合物中相对较大的晶粒尺寸可能归因于W的掺入和/或WO 3诱导的液相烧结,空位辅助了增强的质量传递。我们的发现表明,带隙发生了显着的红移(E g从光吸收光谱可以明显看出),可以实现GWO化合物的宽光谱选择性。W 5d轨道在价带和导带电子之间引起sp – d交换相互作用,这解释了GWO化合物E g的大量红移。同样,随着W的增加,E g线性降低,服从Vegard定律直到x = 0.15,此时,E g突然下降。E g超过x的非线性(弯曲效应)行为= 0.15是由于W在较高浓度下不溶。对合成条件,结构,化学和带隙的相互依赖关系的基本科学理解可能有助于优化用于光学,光电和光催化装置应用的GWO材料。































 京公网安备 11010802027423号
京公网安备 11010802027423号