当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
OH-Initiated Reactions of p-Coumaryl Alcohol Relevant to the Lignin Pyrolysis. Part I. Potential Energy Surface Analysis⊥
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-03-08 00:00:00 , DOI: 10.1021/acs.jpca.9b00185 Rubik Asatryan 1 , Jason M. Hudzik 2 , Joseph W. Bozzelli 2 , Lavrent Khachatryan 3 , Eli Ruckenstein 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-03-08 00:00:00 , DOI: 10.1021/acs.jpca.9b00185 Rubik Asatryan 1 , Jason M. Hudzik 2 , Joseph W. Bozzelli 2 , Lavrent Khachatryan 3 , Eli Ruckenstein 1
Affiliation
|
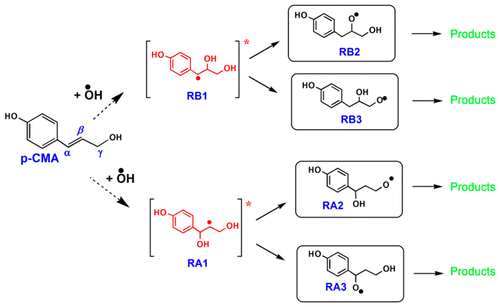
Cinnamyl alcohols such as p-coumaryl alcohol (p-CMA) are lignin models and precursors (monolignols) and the most important primary products of lignin pyrolysis. However, the detection of monomers is not straightforward since they either undergo secondary transformations or repolymerize to contribute to the char formation. Both concerted-molecular and free-radical pathways are involved in these processes. Our recent fundamentally based theoretical and low-temperature matrix-isolation–EPR studies of cinnamyl alcohols highlighted the role of side-chain reactivity in diversity of pyrolysis products and provided a network of the chemically activated H + p-CMA reactions (Asatryan J. Phys. Chem. A, 2017, 121, 3352−3371). The readily available hydroxyl radicals also can trigger a cascade of free-radical processes. Here, we present a comprehensive potential energy surface (PES) analysis of the OH + p-CMA reaction using various DFT and ab initio protocols. Since the p-CMA involves both an alkyl OH-group and a side-chain double bond, the title reaction can also serve as a relevant model for reactions of unsaturated alcohols with hydroxyl radicals to form various oxygenates including polyhydric alcohols which are abundant in nature. The newly identified pathways suggest certain alternatives to the known radical reactions. Of particular interest are the roaming-like low-energy dehydration reactions to generate a variety of O- and C-centered intermediate radicals, which are primarily transformed into the phenolic compounds observed in pyrolysis experiments. Several concerted unimolecular decomposition pathways for p-CMA are also revealed, not considered previously, such as the migration of terminal OH-group, and/or its splitting over the ipso-C and ortho-C atoms of the benzene ring to form bicyclic oxispiro- and chromene compounds represented in natural lignin.
中文翻译:

与木质素热解有关的对-香豆素醇的OH-引发反应。第一部分势能面分析⊥
肉桂醇,例如对-香豆醇(p -CMA)是木质素的模型和前体(单酚),是木质素热解的最重要的主要产物。然而,单体的检测不是直接的,因为它们要么经历二次转化,要么重新聚合以促进炭的形成。这些过程都涉及分子途径和自由基途径。我们最近对肉桂醇进行的基于基础和理论的低温基质分离-EPR研究强调了侧链反应性在热解产物多样性中的作用,并提供了化学活化的H + p- CMA反应网络(Asatryan J.物理 化学 A, 2017, 121,3352-3371)。容易获得的羟基自由基还可引发一系列自由基过程。在这里,我们介绍了使用各种DFT和从头算规程的OH + p- CMA反应的全面势能面(PES)分析。由于p-CMA既涉及烷基OH-基团也涉及侧链双键,该标题反应也可以用作不饱和醇与羟基自由基反应形成各种含氧化合物的反应模型,包括自然界中丰富的多元醇。新近确定的途径表明了已知自由基反应的某些替代方法。特别令人感兴趣的是类似漫游的低能脱水反应,可产生多种以O和C为中心的中间自由基,这些自由基主要转化为热解实验中观察到的酚类化合物。还揭示了先前未曾考虑过的p- CMA的几种协同的单分子分解途径,例如末端OH-基团的迁移和/或其在ipso- C和苯环上的邻位C原子形成天然木质素代表的双环氧化螺环和色烯化合物。
更新日期:2019-03-08
中文翻译:

与木质素热解有关的对-香豆素醇的OH-引发反应。第一部分势能面分析⊥
肉桂醇,例如对-香豆醇(p -CMA)是木质素的模型和前体(单酚),是木质素热解的最重要的主要产物。然而,单体的检测不是直接的,因为它们要么经历二次转化,要么重新聚合以促进炭的形成。这些过程都涉及分子途径和自由基途径。我们最近对肉桂醇进行的基于基础和理论的低温基质分离-EPR研究强调了侧链反应性在热解产物多样性中的作用,并提供了化学活化的H + p- CMA反应网络(













































 京公网安备 11010802027423号
京公网安备 11010802027423号