当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Aerobic Oxidation of 4-Ethylnitrobenzene to 4-Nitroacetophenone Promoted by Metalloporphyrins
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-03-08 00:00:00 , DOI: 10.1021/acs.oprd.9b00030 Yuning Yang 1 , Guijie Li 1 , Xinbiao Mao 1 , Yuanbin She 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-03-08 00:00:00 , DOI: 10.1021/acs.oprd.9b00030 Yuning Yang 1 , Guijie Li 1 , Xinbiao Mao 1 , Yuanbin She 1
Affiliation
|
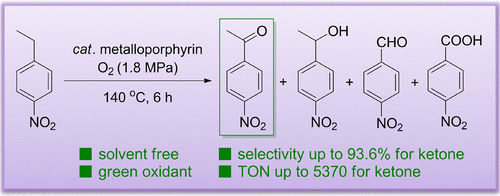
A solvent-free and environment-friendly process for the oxidation of 4-ethylnitrobenzene to 4-nitroacetophenone promoted by metalloporphyrins was developed in a pressure reactor using O2 as a clean oxidant. The activities and reaction selectivities of the metalloporphyrins could be significantly affected by their central metal ions as well as the nature and position of the substituted groups, which were systematically investigated by employing more than 60 metalloporphyrins. Generally, the Fe(III)- and Mn(II)-porphyrins exhibited high activities. Moreover, metalloporphyrins with electron-withdrawing substituents on the para-positions of the phenyl rings showed activities in the order T(p-Br)PPM < T(p-Cl)PPM < T(p-F)PPM. The substituent position effect on the activities of T(o-Cl)PPM > T(m-Cl)PPM > T(p-Cl)PPM and T(o-OMe)PPM < T(m-OMe)PPM < T(p-OMe)PPM were observed. Furthermore, selectivities over 90.0% and a TON of 5370 could be achieved for the desired ketone. Especially, the T(p-Cl)PPMn demonstrated a selectivity of up to 93.6% and a conversion of 51.9% with only 3.3% acid and no alcohol observed, and the selectivity was nearly the same for a large-scale experiment (100 g).
中文翻译:

金属卟啉促进的4-乙基硝基苯选择性好氧氧化成4-硝基苯乙酮
在使用O 2作为清洁氧化剂的压力反应器中,开发了一种无溶剂,环境友好的方法,该方法将金属卟啉催化的4-乙基硝基苯氧化为4-硝基苯乙酮。金属卟啉的活性和反应选择性可能受到其中心金属离子以及取代基团的性质和位置的显着影响,已通过使用60多种金属卟啉进行了系统的研究。通常,Fe(III)-和Mn(II)-卟啉表现出高活性。此外,在苯环对位上具有吸电子取代基的金属卟啉表现出以下活性:T(p -Br)PPM <T(p -Cl)PPM <T(p-F)PPM。取代基位置对T(o -Cl)PPM> T(m -Cl)PPM> T(p -Cl)PPM和T(o -OMe)PPM <T(m -OMe)PPM <T(对-OMe)PPM被观察到。此外,对于期望的酮,可以实现超过90.0%的选择性和5370的TON。尤其是,T(p -Cl)PPMn表现出高达93.6%的选择性和51.9%的转化率,仅观察到3.3%的酸,没有观察到醇,对于大规模实验(100 g )。
更新日期:2019-03-08
中文翻译:

金属卟啉促进的4-乙基硝基苯选择性好氧氧化成4-硝基苯乙酮
在使用O 2作为清洁氧化剂的压力反应器中,开发了一种无溶剂,环境友好的方法,该方法将金属卟啉催化的4-乙基硝基苯氧化为4-硝基苯乙酮。金属卟啉的活性和反应选择性可能受到其中心金属离子以及取代基团的性质和位置的显着影响,已通过使用60多种金属卟啉进行了系统的研究。通常,Fe(III)-和Mn(II)-卟啉表现出高活性。此外,在苯环对位上具有吸电子取代基的金属卟啉表现出以下活性:T(p -Br)PPM <T(p -Cl)PPM <T(p-F)PPM。取代基位置对T(o -Cl)PPM> T(m -Cl)PPM> T(p -Cl)PPM和T(o -OMe)PPM <T(m -OMe)PPM <T(对-OMe)PPM被观察到。此外,对于期望的酮,可以实现超过90.0%的选择性和5370的TON。尤其是,T(p -Cl)PPMn表现出高达93.6%的选择性和51.9%的转化率,仅观察到3.3%的酸,没有观察到醇,对于大规模实验(100 g )。































 京公网安备 11010802027423号
京公网安备 11010802027423号