Nature Communications ( IF 14.7 ) Pub Date : 2019-03-08 , DOI: 10.1038/s41467-019-09098-w Linda Celeste Montemiglio , Claudia Testi , Pierpaolo Ceci , Elisabetta Falvo , Martina Pitea , Carmelinda Savino , Alessandro Arcovito , Giovanna Peruzzi , Paola Baiocco , Filippo Mancia , Alberto Boffi , Amédée des Georges , Beatrice Vallone
|
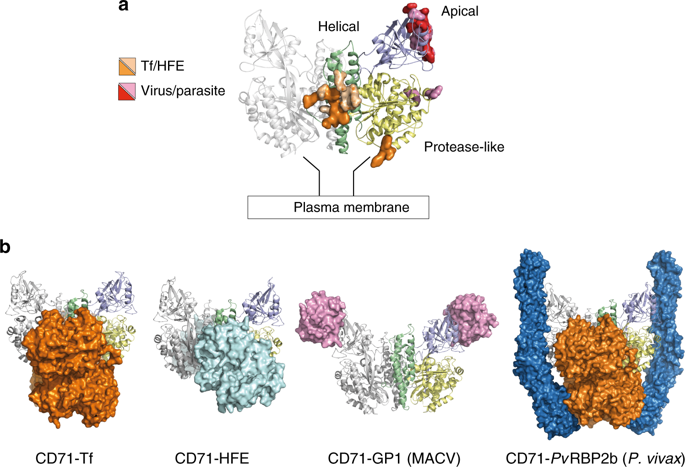
Human transferrin receptor 1 (CD71) guarantees iron supply by endocytosis upon binding of iron-loaded transferrin and ferritin. Arenaviruses and the malaria parasite exploit CD71 for cell invasion and epitopes on CD71 for interaction with transferrin and pathogenic hosts were identified. Here, we provide the molecular basis of the CD71 ectodomain-human ferritin interaction by determining the 3.9 Å resolution single-particle cryo-electron microscopy structure of their complex and by validating our structural findings in a cellular context. The contact surfaces between the heavy-chain ferritin and CD71 largely overlap with arenaviruses and Plasmodium vivax binding regions in the apical part of the receptor ectodomain. Our data account for transferrin-independent binding of ferritin to CD71 and suggest that select pathogens may have adapted to enter cells by mimicking the ferritin access gate.
中文翻译:

人类铁蛋白-转铁蛋白受体1复合体的Cryo-EM结构
人运铁蛋白受体1(CD71)在载铁的运铁蛋白和铁蛋白结合后通过胞吞作用保证铁的供应。鉴定了无粒病毒和疟疾寄生虫利用CD71进行细胞侵袭,并鉴定了CD71上的表位与运铁蛋白和致病宿主相互作用。在这里,我们通过确定其复合物的3.9Å分辨率单粒子冷冻电子显微镜结构并在细胞环境中验证我们的结构发现,为CD71胞外域-人铁蛋白相互作用提供了分子基础。重链铁蛋白和CD71之间的接触表面与沙粒病毒和间日疟原虫大量重叠受体胞外域顶部的结合区域。我们的数据解释了铁蛋白与CD71的转铁蛋白非依赖性结合,并表明某些病原体可能已经通过模仿铁蛋白进入门而适应进入细胞。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号