European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-03-08 , DOI: 10.1016/j.ejmech.2019.03.006 Wei Zhao , Long He , Tian-Le Xiang , Ya-Jie Tang
|
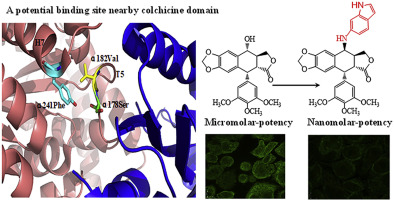
The colchicine binding site of tubulin is an attractive molecular target domain for cancer therapies. However, there was no FDA approved drug for targeting colchicine domain. Our previous crystallography discovered that a potential binding site of αT5 loop-αH7 nearby colchicine domain was beneficial for introducing affinity fragment. In this work, benzo heterocycles (i.e., indole, indazole and quinoline) with the high affinity ability of αT5 loop-αH7 were chosen as affinity fragment to modify the molecule structure of podophyllotoxin for improving the tubulin binding affinity. 4β-NH-(benzo heterocycles)-4-desoxy-podophyllotoxin were synchronously located at α/β interface of tubulin through providing affinity fragment to αT5 loop-αH7 (i.e., α178Ser, α182Val, α241Phe) and colchicine domain (i.e., β241Cys, β124ASP). 4β-NH-(6″-aminoindole)-4-desoxy-podophyllotoxin not only exhibited nanomolar antitumor potency in vitro but also destroyed solid tumor growth without lethal toxicity in vivo. The correctness of rational drug design was strictly demonstrated by bioactivity test.
中文翻译:

查询4β- NH - (6-氨基吲哚)-4-脱氧鬼臼毒素以纳摩尔-效力的抗肿瘤活性通过提高微管蛋白的电位的基础上结合亲和力结合位点附近的秋水仙素域
微管蛋白的秋水仙碱结合位点是用于癌症治疗的有吸引力的分子靶结构域。但是,尚无FDA批准的靶向秋水仙碱域的药物。我们以前的晶体学发现,秋水仙碱结构域附近的αT5环-αH7的潜在结合位点有利于引入亲和片段。在这项工作中,选择具有高αT5环-αH7亲和力的苯并杂环(即吲哚,吲唑和喹啉)作为亲和片段,以修饰鬼臼毒素的分子结构,从而改善微管蛋白的结合亲和力。4β- NH通过向αT5环-αH7(即,α178Ser,α182Val,α241Phe)和秋水仙碱域(即,β241Cys,β124ASP)提供亲和力片段,将-(苯并杂环)-4-脱氧鬼臼毒素同步定位在微管蛋白的α/β界面。4β- NH-(6″-氨基吲哚)-4-脱氧鬼臼毒素不仅在体外表现出纳摩尔级的抗肿瘤能力,而且在体内不破坏致命毒性的情况下也破坏了实体瘤的生长。通过生物活性测试严格证明了合理药物设计的正确性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号