European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-03-08 , DOI: 10.1016/j.ejmech.2019.03.003 Bin Liu 1 , Xia Yuan 1 , Bo Xu 1 , Han Zhang 1 , Ridong Li 2 , Xin Wang 1 , Zemei Ge 1 , Runtao Li 1
|
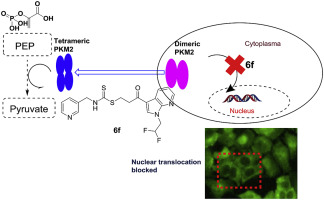
Multiple lines of evidence have indicated that pyruvate kinase M2 (PKM2) is upregulated in most cancer cells and it is increasingly recognized as a potential therapeutic target in oncology. In a continuation of our discovery of lead compound 5 and SAR study, the 7-azaindole moiety in compound 5 was systematically optimized. The results showed that compound 6f, which has a difluoroethyl substitution on the 7-azaindole ring, exhibited high PKM2 activation potency and anti-proliferation activities on A375 cell lines. In a xenograft mouse model, oral administration of compound 6f led to significant tumor regression without obvious toxicity. Further mechanistic studies revealed that 6f could influence the translocation of PKM2 into nucleus, as well as induction of apoptosis and autophagy of A375 cells. More importantly, compound 6f significantly inhibited migration of A375 cells in a concentration-dependent manner. Collectively, 6f may serve as a lead compound in the development of potent PKM2 activators for cancer therapy.
中文翻译:

含有吡啶-3-基甲基二硫代氨基甲酸酯部分的新型 7-氮杂吲哚衍生物的合成,作为有效的 PKM2 激活剂和 PKM2 核易位抑制剂
多种证据表明丙酮酸激酶 M2 (PKM2) 在大多数癌细胞中表达上调,并且越来越多地被认为是肿瘤学中的潜在治疗靶点。在我们先导化合物5的发现和 SAR 研究的延续中,化合物5中的 7-氮杂吲哚部分得到了系统优化。结果表明,在7-氮杂吲哚环上具有二氟乙基取代的化合物6f对A375细胞系表现出高PKM2激活效力和抗增殖活性。在异种移植小鼠模型中,口服化合物6f导致肿瘤显着消退,且没有明显毒性。进一步的机制研究表明, 6f可以影响PKM2转入细胞核,并诱导A375细胞凋亡和自噬。更重要的是,化合物6f以浓度依赖性方式显着抑制A375细胞的迁移。总的来说, 6f可以作为开发用于癌症治疗的有效 PKM2 激活剂的先导化合物。































 京公网安备 11010802027423号
京公网安备 11010802027423号