当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Formate from CO2 Gas Catalyzed by an O2-Tolerant NAD-Dependent Formate Dehydrogenase and Glucose Dehydrogenase.
Biochemistry ( IF 2.9 ) Pub Date : 2019-03-18 , DOI: 10.1021/acs.biochem.8b01301 Xuejun Yu , Dimitri Niks , Xin Ge , Haizhou Liu , Russ Hille , Ashok Mulchandani
Biochemistry ( IF 2.9 ) Pub Date : 2019-03-18 , DOI: 10.1021/acs.biochem.8b01301 Xuejun Yu , Dimitri Niks , Xin Ge , Haizhou Liu , Russ Hille , Ashok Mulchandani
|
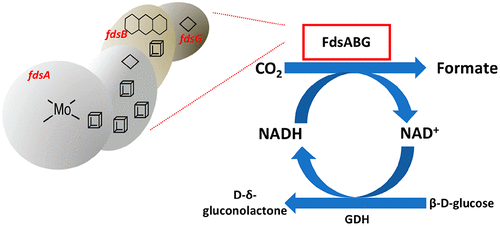
Direct biocatalytic conversion of CO2 to formic acid is an attractive means of reversibly storing energy in chemical bonds. Formate dehydrogenases (FDHs) are a heterogeneous group of enzymes that catalyze the oxidation of formic acid to carbon dioxide, generating two protons and two electrons. Several FDHs have recently been reported to catalyze the reverse reaction, i.e., the reduction of carbon dioxide to formic acid, under appropriate conditions. The main challenges with these enzymes are relatively low rates of CO2 reduction and high oxygen sensitivity. Our earlier studies (Yu et al. (2017) J. Biol. Chem. 292, 16872-16879) have shown that the FdsABG formate dehydrogenase from Cupriavidus necator is able to effectively catalyze the reduction of CO2, using NADH as a source of reducing equivalents, with a good oxygen tolerance. On the basis of this result, we have developed a highly thermodynamically efficient and cost-effective biocatalytic process for the transformation of CO2 to formic acid using FdsABG. We have cloned the full-length soluble formate dehydrogenase (FdsABG) from C. necator and expressed it in Escherichia coli with a His-tag fused to the N terminus of the FdsG subunit; this overexpression system has greatly simplified the FdsABG purification process. Importantly, we have also combined this recombinant C. necator FdsABG with another enzyme, glucose dehydrogenase, for continuous regeneration of NADH for CO2 reduction and demonstrated that the combined system is highly effective in reducing CO2 to formate. The results indicate that this system shows significant promise for the future development of an enzyme-based system for the industrial reduction of CO2.
中文翻译:

耐O2的NAD依赖性甲酸酯脱氢酶和葡萄糖脱氢酶催化从CO2气体合成甲酸酯。
将CO2直接生物催化转化为甲酸是一种可逆地以化学键存储能量的吸引人的方法。甲酰胺脱氢酶(FDHs)是一种异质酶,可催化甲酸氧化为二氧化碳,产生两个质子和两个电子。最近报道了几种FDH在合适的条件下催化逆反应,即将二氧化碳还原为甲酸。这些酶的主要挑战是相对较低的CO 2还原速率和较高的氧敏感性。我们较早的研究(Yu et al。(2017)J.Biol.Chem.292,16872-16879)表明,使用NADH作为还原源,来自Cupriavidus necator的FdsABG甲酸脱氢酶能够有效催化CO2的还原。当量,具有良好的耐氧性。基于此结果,我们开发了一种高度热力学有效且具有成本效益的生物催化方法,用于使用FdsABG将CO2转化为甲酸。我们已经从C. necator克隆了全长可溶性甲酸脱氢酶(FdsABG),并在大肠杆菌中表达了带有与FdsG亚基N末端融合的His标签的大肠杆菌。这种过表达系统大大简化了FdsABG纯化过程。重要的是,我们还将这种重组梭状芽胞杆菌FdsABG与另一种酶(葡萄糖脱氢酶)结合使用,以连续再生NADH以减少CO2,并证明了该组合系统在将CO2还原成甲酸酯方面非常有效。
更新日期:2019-03-06
中文翻译:

耐O2的NAD依赖性甲酸酯脱氢酶和葡萄糖脱氢酶催化从CO2气体合成甲酸酯。
将CO2直接生物催化转化为甲酸是一种可逆地以化学键存储能量的吸引人的方法。甲酰胺脱氢酶(FDHs)是一种异质酶,可催化甲酸氧化为二氧化碳,产生两个质子和两个电子。最近报道了几种FDH在合适的条件下催化逆反应,即将二氧化碳还原为甲酸。这些酶的主要挑战是相对较低的CO 2还原速率和较高的氧敏感性。我们较早的研究(Yu et al。(2017)J.Biol.Chem.292,16872-16879)表明,使用NADH作为还原源,来自Cupriavidus necator的FdsABG甲酸脱氢酶能够有效催化CO2的还原。当量,具有良好的耐氧性。基于此结果,我们开发了一种高度热力学有效且具有成本效益的生物催化方法,用于使用FdsABG将CO2转化为甲酸。我们已经从C. necator克隆了全长可溶性甲酸脱氢酶(FdsABG),并在大肠杆菌中表达了带有与FdsG亚基N末端融合的His标签的大肠杆菌。这种过表达系统大大简化了FdsABG纯化过程。重要的是,我们还将这种重组梭状芽胞杆菌FdsABG与另一种酶(葡萄糖脱氢酶)结合使用,以连续再生NADH以减少CO2,并证明了该组合系统在将CO2还原成甲酸酯方面非常有效。































 京公网安备 11010802027423号
京公网安备 11010802027423号