当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CO2 Capture from Flue Gas Using an Electrochemically Reversible Hydroquinone/Quinone Solution
Energy & Fuels ( IF 5.2 ) Pub Date : 2019-03-06 00:00:00 , DOI: 10.1021/acs.energyfuels.8b04419 Chuanliang Huang 1 , Changjun Liu 1 , Kejing Wu 2 , Hairong Yue 1 , Siyang Tang 1 , Houfang Lu 1, 2 , Bin Liang 1, 2
Energy & Fuels ( IF 5.2 ) Pub Date : 2019-03-06 00:00:00 , DOI: 10.1021/acs.energyfuels.8b04419 Chuanliang Huang 1 , Changjun Liu 1 , Kejing Wu 2 , Hairong Yue 1 , Siyang Tang 1 , Houfang Lu 1, 2 , Bin Liang 1, 2
Affiliation
|
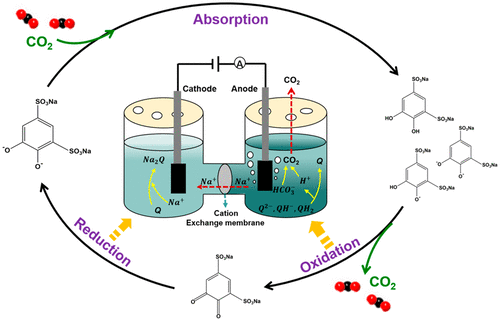
Electrochemical methods are potentially an energy-saving way to capture CO2 from flue gas. Unlike the regeneration of amine through high-temperature thermal distillation in commercial CO2 absorption processes, the CO2 absorption solution is regenerated by electrolysis at a low temperature. In this work, tiron (disodium 4,5-dihydroxy-1,3-benzenedisulfonate, QH2) was employed as a pH mediator because its redox reactions can change the pH of the solution. Na2Q, which was prepared by QH2 and NaOH in a molar ratio of 1:2, was used to capture CO2 because of its alkalinity. Then, CO2 was desorbed by the oxidation of QH– and QH2 formed in the CO2-rich aqueous solution to quinone (Q) to release protons, and the alkalinity was recovered by the reduction of Q to the quinone dianion (Q2–). The redox performance of Na2Q in aqueous solution was investigated using cyclic voltammetry, and the CO2 capacity of Na2Q solutions at different concentrations (0.1–0.7 M) was measured. The results show that the redox behavior of Na2Q was reversible in the neutral or weakly alkaline solutions. However, the reduction of Q to Q2– by electrolysis was difficult in a high pH solution. During adsorption, the Na2Q solution absorbed CO2 at a molar ratio of about 1:1 (CO2/Q2– ≈ 1). The CO2-saturated Na2Q solution was electrolyzed in the anode zone under a constant current. The CO2 desorption rate reached 100%, and Q2–, QH–, and QH2 were oxidized to give Q. In the cathode zone, Q was reduced to Q2–, which could be used to adsorb CO2 from flue gas. On the basis of the potential difference between the cathode and anode, the regeneration energy consumption was estimated to be about 2.4 GJ/tonne of CO2.
中文翻译:

使用电化学可逆对苯二酚/醌溶液从烟气中捕获CO 2
电化学方法可能是从烟气中捕获CO 2的一种节能方式。与在商业CO 2吸收过程中通过高温热蒸馏来再生胺不同,CO 2吸收溶液是通过在低温下电解来再生的。在这项工作中,将铁(4,5-二羟基-1,3-苯二磺酸二钠,QH 2)用作pH介质,因为其氧化还原反应可以改变溶液的pH。由QH 2和NaOH以1:2的摩尔比制备的Na 2 Q由于其碱度而被用于捕获CO 2。然后,CO 2通过QH的氧化而解吸–在富CO 2水溶液中形成QH 2并生成醌(Q)以释放质子,并通过将Q还原为醌二价阴离子(Q 2–)来恢复碱度。使用循环伏安法研究了水溶液中Na 2 Q的氧化还原性能,并测量了不同浓度(0.1–0.7 M)下Na 2 Q溶液的CO 2容量。结果表明,Na 2 Q的氧化还原行为在中性或弱碱性溶液中是可逆的。但是,在高pH值的溶液中,通过电解将Q还原为Q 2–很难。在吸附过程中,Na 2 Q溶液吸收了CO2的摩尔比约为1:1(CO 2 / Q 2– ≈1)。在恒定电流下在阳极区中电解饱和CO 2的Na 2 Q溶液。CO 2的解吸率达到100%,并且将Q 2–,QH –和QH 2氧化为Q。在阴极区,Q还原为Q 2–,可用于从烟道气中吸附CO 2。 。基于阴极和阳极之间的电势差,估计再生能量消耗为约2.4GJ /吨CO 2。
更新日期:2019-03-06
中文翻译:

使用电化学可逆对苯二酚/醌溶液从烟气中捕获CO 2
电化学方法可能是从烟气中捕获CO 2的一种节能方式。与在商业CO 2吸收过程中通过高温热蒸馏来再生胺不同,CO 2吸收溶液是通过在低温下电解来再生的。在这项工作中,将铁(4,5-二羟基-1,3-苯二磺酸二钠,QH 2)用作pH介质,因为其氧化还原反应可以改变溶液的pH。由QH 2和NaOH以1:2的摩尔比制备的Na 2 Q由于其碱度而被用于捕获CO 2。然后,CO 2通过QH的氧化而解吸–在富CO 2水溶液中形成QH 2并生成醌(Q)以释放质子,并通过将Q还原为醌二价阴离子(Q 2–)来恢复碱度。使用循环伏安法研究了水溶液中Na 2 Q的氧化还原性能,并测量了不同浓度(0.1–0.7 M)下Na 2 Q溶液的CO 2容量。结果表明,Na 2 Q的氧化还原行为在中性或弱碱性溶液中是可逆的。但是,在高pH值的溶液中,通过电解将Q还原为Q 2–很难。在吸附过程中,Na 2 Q溶液吸收了CO2的摩尔比约为1:1(CO 2 / Q 2– ≈1)。在恒定电流下在阳极区中电解饱和CO 2的Na 2 Q溶液。CO 2的解吸率达到100%,并且将Q 2–,QH –和QH 2氧化为Q。在阴极区,Q还原为Q 2–,可用于从烟道气中吸附CO 2。 。基于阴极和阳极之间的电势差,估计再生能量消耗为约2.4GJ /吨CO 2。













































 京公网安备 11010802027423号
京公网安备 11010802027423号