Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Serendipitous Formation of 2H-Pyrazolo[3,4-d]pyridazin-7(6H)-ones from 3-Arylsydnones
ACS Omega ( IF 3.7 ) Pub Date : 2019-03-06 00:00:00 , DOI: 10.1021/acsomega.8b02013 Mahadev N. Kumbar 1 , Saba Kauser J. Shaikh 1 , Ravindra R. Kamble 1 , Praveen K. Bayannavar 1 , Atulkumar A. Kamble 1 , Raveendra K. Hunnur 2
ACS Omega ( IF 3.7 ) Pub Date : 2019-03-06 00:00:00 , DOI: 10.1021/acsomega.8b02013 Mahadev N. Kumbar 1 , Saba Kauser J. Shaikh 1 , Ravindra R. Kamble 1 , Praveen K. Bayannavar 1 , Atulkumar A. Kamble 1 , Raveendra K. Hunnur 2
Affiliation
|
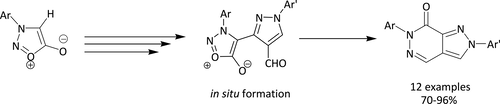
Fused nitrogen heterocyclesnamely, pyrazolo[3,4-d]pyridazin-7(6H)-ones have been obtained by exploiting the 1,3-dipolar nature of N-arylsydnones, from hydrazones of 3-aryl-4-acetylsydnones via the Vilsmeier–Haack strategy. Facile intramolecular nucleophilic addition followed by CO2 elimination under reflux or upon microwave irradiation was presented. Plausible mechanisms for the formation of the title compounds are proffered. Structure confirmatory evidence came from single-crystal X-ray crystallography.
中文翻译:

由3-芳基sydnones偶然形成2 H -Pyrazolo [3,4- d ] pyridazin -7(6 H)-ones
稠合氮heterocyclesnamely,吡唑并[3,4- d ]哒嗪-7-(6 ħ) -酮已通过开发的1,3-偶极性质得到 Ñ -arylsydnones,从3-芳基-4- acetylsydnones腙经由所述Vilsmeier–Haack策略。提出了容易的分子内亲核加成,然后在回流下或微波辐射下消除CO 2的方法。提出了可能的标题化合物形成机理。结构确认证据来自单晶X射线晶体学。
更新日期:2019-03-06
中文翻译:

由3-芳基sydnones偶然形成2 H -Pyrazolo [3,4- d ] pyridazin -7(6 H)-ones
稠合氮heterocyclesnamely,吡唑并[3,4- d ]哒嗪-7-(6 ħ) -酮已通过开发的1,3-偶极性质得到 Ñ -arylsydnones,从3-芳基-4- acetylsydnones腙经由所述Vilsmeier–Haack策略。提出了容易的分子内亲核加成,然后在回流下或微波辐射下消除CO 2的方法。提出了可能的标题化合物形成机理。结构确认证据来自单晶X射线晶体学。































 京公网安备 11010802027423号
京公网安备 11010802027423号