Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural basis of STING binding with and phosphorylation by TBK1
Nature ( IF 50.5 ) Pub Date : 2019-03-01 , DOI: 10.1038/s41586-019-1000-2 Conggang Zhang 1 , Guijun Shang 2 , Xiang Gui 1 , Xuewu Zhang 2, 3 , Xiao-Chen Bai 3, 4 , Zhijian J Chen 1, 5, 6
Nature ( IF 50.5 ) Pub Date : 2019-03-01 , DOI: 10.1038/s41586-019-1000-2 Conggang Zhang 1 , Guijun Shang 2 , Xiang Gui 1 , Xuewu Zhang 2, 3 , Xiao-Chen Bai 3, 4 , Zhijian J Chen 1, 5, 6
Affiliation
|
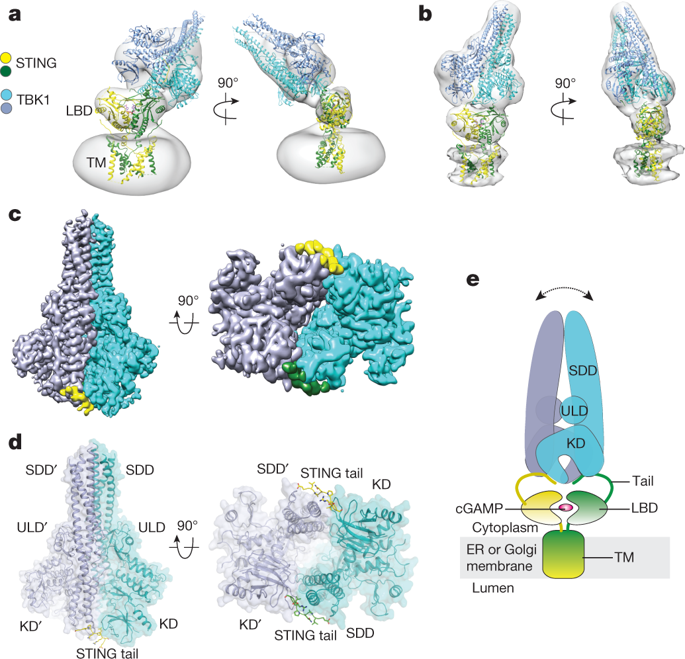
The invasion of mammalian cytoplasm by microbial DNA from infectious pathogens or by self DNA from the nucleus or mitochondria represents a danger signal that alerts the host immune system1. Cyclic GMP–AMP synthase (cGAS) is a sensor of cytoplasmic DNA that activates the type-I interferon pathway2. On binding to DNA, cGAS is activated to catalyse the synthesis of cyclic GMP–AMP (cGAMP) from GTP and ATP3. cGAMP functions as a second messenger that binds to and activates stimulator of interferon genes (STING)3–9. STING then recruits and activates tank-binding kinase 1 (TBK1), which phosphorylates STING and the transcription factor IRF3 to induce type-I interferons and other cytokines10,11. However, how cGAMP-bound STING activates TBK1 and IRF3 is not understood. Here we present the cryo-electron microscopy structure of human TBK1 in complex with cGAMP-bound, full-length chicken STING. The structure reveals that the C-terminal tail of STING adopts a β-strand-like conformation and inserts into a groove between the kinase domain of one TBK1 subunit and the scaffold and dimerization domain of the second subunit in the TBK1 dimer. In this binding mode, the phosphorylation site Ser366 in the STING tail cannot reach the kinase-domain active site of bound TBK1, which suggests that STING phosphorylation by TBK1 requires the oligomerization of both proteins. Mutational analyses validate the interaction mode between TBK1 and STING and support a model in which high-order oligomerization of STING and TBK1, induced by cGAMP, leads to STING phosphorylation by TBK1.The cryo-electron microscopy structure of human TBK1 in complex with cyclic GMP–AMP-bound chicken STING reveals a binding mode that suggests that STING phosphorylation by TBK1 requires oligomerization of both proteins.
中文翻译:

STING 与 TBK1 结合和磷酸化的结构基础
来自传染性病原体的微生物 DNA 或来自细胞核或线粒体的自身 DNA 对哺乳动物细胞质的入侵代表了警告宿主免疫系统的危险信号。环 GMP-AMP 合酶 (cGAS) 是细胞质 DNA 的传感器,可激活 I 型干扰素途径 2。与 DNA 结合后,cGAS 被激活以催化 GTP 和 ATP3 合成环 GMP-AMP (cGAMP)。cGAMP 作为第二信使结合并激活干扰素基因 (STING)3-9 的刺激物。然后 STING 募集并激活坦克结合激酶 1 (TBK1),后者使 STING 和转录因子 IRF3 磷酸化以诱导 I 型干扰素和其他细胞因子10,11。然而,cGAMP 结合的 STING 如何激活 TBK1 和 IRF3 尚不清楚。在这里,我们展示了人类 TBK1 与 cGAMP 结合的全长鸡 STING 复合物的冷冻电子显微镜结构。该结构表明,STING 的 C 端尾部采用 β 链样构象并插入 TBK1 二聚体中一个亚基的激酶结构域与第二个亚基的支架和二聚化结构域之间的凹槽中。在这种结合模式下,STING 尾部的磷酸化位点 Ser366 无法到达结合的 TBK1 的激酶结构域活性位点,这表明 TBK1 对 STING 的磷酸化需要两种蛋白质的寡聚化。突变分析验证了 TBK1 和 STING 之间的相互作用模式,并支持 cGAMP 诱导的 STING 和 TBK1 的高阶寡聚化导致 TBK1 磷酸化 STING 的模型。
更新日期:2019-03-01
中文翻译:

STING 与 TBK1 结合和磷酸化的结构基础
来自传染性病原体的微生物 DNA 或来自细胞核或线粒体的自身 DNA 对哺乳动物细胞质的入侵代表了警告宿主免疫系统的危险信号。环 GMP-AMP 合酶 (cGAS) 是细胞质 DNA 的传感器,可激活 I 型干扰素途径 2。与 DNA 结合后,cGAS 被激活以催化 GTP 和 ATP3 合成环 GMP-AMP (cGAMP)。cGAMP 作为第二信使结合并激活干扰素基因 (STING)3-9 的刺激物。然后 STING 募集并激活坦克结合激酶 1 (TBK1),后者使 STING 和转录因子 IRF3 磷酸化以诱导 I 型干扰素和其他细胞因子10,11。然而,cGAMP 结合的 STING 如何激活 TBK1 和 IRF3 尚不清楚。在这里,我们展示了人类 TBK1 与 cGAMP 结合的全长鸡 STING 复合物的冷冻电子显微镜结构。该结构表明,STING 的 C 端尾部采用 β 链样构象并插入 TBK1 二聚体中一个亚基的激酶结构域与第二个亚基的支架和二聚化结构域之间的凹槽中。在这种结合模式下,STING 尾部的磷酸化位点 Ser366 无法到达结合的 TBK1 的激酶结构域活性位点,这表明 TBK1 对 STING 的磷酸化需要两种蛋白质的寡聚化。突变分析验证了 TBK1 和 STING 之间的相互作用模式,并支持 cGAMP 诱导的 STING 和 TBK1 的高阶寡聚化导致 TBK1 磷酸化 STING 的模型。































 京公网安备 11010802027423号
京公网安备 11010802027423号