当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
B(C6F5)3‐Enabled Synthesis of a Cyclic cis‐Arsaphosphene
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-03-27 , DOI: 10.1002/chem.201901022
Meera Mehta 1 , John E. McGrady 1 , Jose M. Goicoechea 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-03-27 , DOI: 10.1002/chem.201901022
Meera Mehta 1 , John E. McGrady 1 , Jose M. Goicoechea 1
Affiliation
|
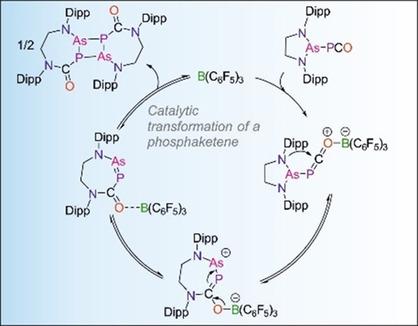
The synthesis and characterization of an (arsino)phosphaketene, As(PCO){[N(Dipp)](CH2)}2 (Dipp=2,6‐diisopropylphenyl) is reported along with its subsequent reactivity with B(C6F5)3. When reacted in a stoichiometric ratio, B(C6F5)3 drove the insertion of the P=C bond of the phosphaketene into one of the As−N bonds of the arsino functionality, affording an acid‐stabilized, seven‐membered, cyclic arsaphosphene. In contrast, when catalytic amounts of B(C6F5)3 were employed, dimeric species, which formed through a formal [2+2] cycloaddition of the cyclic arsaphosphene, were generated. The cyclic arsaphosphene product represents the first example of such a compound in which the two substituents are arranged in a cis‐configuration.
中文翻译:

B(C6F5)3使能的环状顺式Arsaphosphene的合成
报告了(arsino)磷烯(As(PCO){[N(Dipp)](CH 2)} 2(Dipp = 2,6-二异丙基苯基)的合成和表征,以及其随后与B(C 6 F 5)3。当以化学计量比进行反应时,B(C 6 F 5)3驱使磷烯酮的P = C键插入到Arsino官能团的As-N键之一中,从而提供了酸稳定的七元原子,环状亚磷phosph。相反,当催化量的B(C 6 F 5)3当使用二聚体时,生成了二聚体,该二聚体是通过环状砷化膦的正式[2 + 2]环加成反应而形成的。环状a磷鎓产物代表了这样一个化合物的第一个例子,其中两个取代基排列成顺式构型。
更新日期:2019-03-27
中文翻译:

B(C6F5)3使能的环状顺式Arsaphosphene的合成
报告了(arsino)磷烯(As(PCO){[N(Dipp)](CH 2)} 2(Dipp = 2,6-二异丙基苯基)的合成和表征,以及其随后与B(C 6 F 5)3。当以化学计量比进行反应时,B(C 6 F 5)3驱使磷烯酮的P = C键插入到Arsino官能团的As-N键之一中,从而提供了酸稳定的七元原子,环状亚磷phosph。相反,当催化量的B(C 6 F 5)3当使用二聚体时,生成了二聚体,该二聚体是通过环状砷化膦的正式[2 + 2]环加成反应而形成的。环状a磷鎓产物代表了这样一个化合物的第一个例子,其中两个取代基排列成顺式构型。

































 京公网安备 11010802027423号
京公网安备 11010802027423号