当前位置:
X-MOL 学术
›
Nat. Rev. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interplay between β-lactamases and new β-lactamase inhibitors.
Nature Reviews Microbiology ( IF 69.2 ) Pub Date : 2019-05-01 , DOI: 10.1038/s41579-019-0159-8 Karen Bush 1 , Patricia A Bradford 2
Nature Reviews Microbiology ( IF 69.2 ) Pub Date : 2019-05-01 , DOI: 10.1038/s41579-019-0159-8 Karen Bush 1 , Patricia A Bradford 2
Affiliation
|
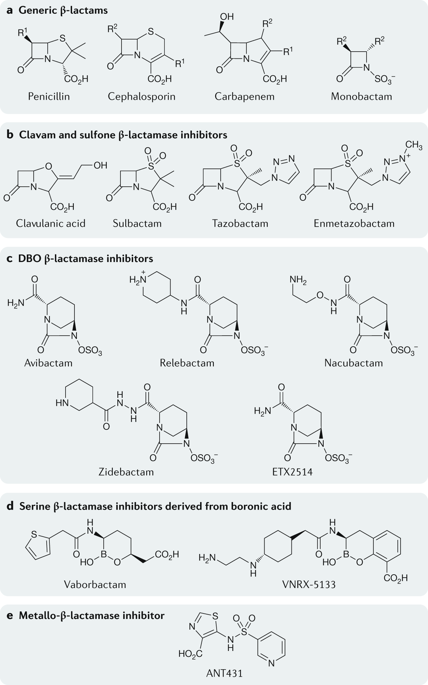
Resistance to β-lactam antibiotics in Gram-negative bacteria is commonly associated with production of β-lactamases, including extended-spectrum β-lactamases (ESBLs) and carbapenemases belonging to different molecular classes: those with a catalytically active serine and those with at least one active-site Zn2+ to facilitate hydrolysis. To counteract the hydrolytic activity of these enzymes, combinations of a β-lactam with a β-lactamase inhibitor (BLI) have been clinically successful. However, some β-lactam-BLI combinations have lost their effectiveness against prevalent Gram-negative pathogens that produce ESBLs, carbapenemases or multiple β-lactamases in the same organism. In this Review, descriptions are provided for medically relevant β-lactamase families and various BLI combinations that have been developed or are under development. Recently approved inhibitor combinations include the inhibitors avibactam and vaborbactam of the diazabicyclooctanone and boronic acid inhibitor classes, respectively, as new scaffolds for future inhibitor design.
中文翻译:

β-内酰胺酶和新的β-内酰胺酶抑制剂之间的相互作用。
革兰氏阴性菌对 β-内酰胺类抗生素的耐药性通常与 β-内酰胺酶的产生有关,包括属于不同分子类别的超广谱 β-内酰胺酶 (ESBL) 和碳青霉烯酶:具有催化活性丝氨酸的那些和至少具有催化活性的那些一个活性位点 Zn2+ 以促进水解。为了抵消这些酶的水解活性,β-内酰胺与 β-内酰胺酶抑制剂 (BLI) 的组合已在临床上取得成功。然而,一些 β-内酰胺-BLI 组合对在同一生物体中产生 ESBL、碳青霉烯酶或多种 β-内酰胺酶的流行革兰氏阴性病原体失去了效力。在这篇综述中,描述了医学相关的 β-内酰胺酶家族和已经开发或正在开发的各种 BLI 组合。
更新日期:2019-03-06
中文翻译:

β-内酰胺酶和新的β-内酰胺酶抑制剂之间的相互作用。
革兰氏阴性菌对 β-内酰胺类抗生素的耐药性通常与 β-内酰胺酶的产生有关,包括属于不同分子类别的超广谱 β-内酰胺酶 (ESBL) 和碳青霉烯酶:具有催化活性丝氨酸的那些和至少具有催化活性的那些一个活性位点 Zn2+ 以促进水解。为了抵消这些酶的水解活性,β-内酰胺与 β-内酰胺酶抑制剂 (BLI) 的组合已在临床上取得成功。然而,一些 β-内酰胺-BLI 组合对在同一生物体中产生 ESBL、碳青霉烯酶或多种 β-内酰胺酶的流行革兰氏阴性病原体失去了效力。在这篇综述中,描述了医学相关的 β-内酰胺酶家族和已经开发或正在开发的各种 BLI 组合。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号