当前位置:
X-MOL 学术
›
ACS Comb. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Hydantoin and Diketopiperazine-Fused Tetrahydroisoquinolines via Pictet-Spengler Reaction.
ACS Combinatorial Science Pub Date : 2019-03-06 , DOI: 10.1021/acscombsci.9b00005 Shih-I Liu,Jia-Yun Haung,Indrajeet J Barve,Sheng-Cih Huang,Chung-Ming Sun
ACS Combinatorial Science Pub Date : 2019-03-06 , DOI: 10.1021/acscombsci.9b00005 Shih-I Liu,Jia-Yun Haung,Indrajeet J Barve,Sheng-Cih Huang,Chung-Ming Sun
|
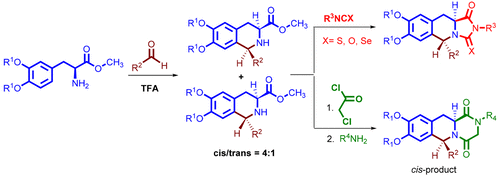
An enantioselective synthesis of iso-, isothio-, and isoselenohydantoin and diketopiperazine-fused tetrahydroisoquinolines from l-Dopa was reported. The route consists of an Pictet-Spengler reaction of ( S)-2-amino-3-(3,4-dimethoxyphenyl)propanoates with various aldehydes to afford diastereomeric tetrahydroisoquinolines. Next step, the tetrahydroisoquinolines were further reacted with iso-, isothio-, or isoselenocyanates to construct hydantoin. Similarly, the diketopiperazine moiety was constructed by subjecting tetrahydroisoquinolines to a condensation reaction with chloroacetyl chloride followed by nucleophilic addition with various primary amines.
中文翻译:

通过Pictet-Spengler反应对乙内酰脲和二酮哌嗪融合的四氢异喹啉进行对映选择性合成。
据报道,从I-Dopa对映异构合成异-,异硫-和异亚硒基乙内酰脲和二酮哌嗪融合的四氢异喹啉。该路线由(S)-2-氨基-3-(3,4-二甲氧基苯基)丙酸酯与各种醛的Pictet-Spengler反应组成,以提供非对映体四氢异喹啉。下一步,使四氢异喹啉与异氰酸酯,异硫氰酸酯或异鲸蜡氰酸酯进一步反应以构建乙内酰脲。类似地,通过使四氢异喹啉与氯乙酰氯进行缩合反应,然后与各种伯胺进行亲核加成,来构建二酮哌嗪部分。
更新日期:2019-03-06
中文翻译:

通过Pictet-Spengler反应对乙内酰脲和二酮哌嗪融合的四氢异喹啉进行对映选择性合成。
据报道,从I-Dopa对映异构合成异-,异硫-和异亚硒基乙内酰脲和二酮哌嗪融合的四氢异喹啉。该路线由(S)-2-氨基-3-(3,4-二甲氧基苯基)丙酸酯与各种醛的Pictet-Spengler反应组成,以提供非对映体四氢异喹啉。下一步,使四氢异喹啉与异氰酸酯,异硫氰酸酯或异鲸蜡氰酸酯进一步反应以构建乙内酰脲。类似地,通过使四氢异喹啉与氯乙酰氯进行缩合反应,然后与各种伯胺进行亲核加成,来构建二酮哌嗪部分。













































 京公网安备 11010802027423号
京公网安备 11010802027423号