当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tris(pentafluorophenyl)borane‐Assisted Chiral Phosphoric Acid Catalysts for Enantioselective Inverse‐Electron‐Demand Hetero‐Diels‐Alder Reaction of α,β‐Substituted Acroleins
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2019-04-01 , DOI: 10.1002/ajoc.201900104 Manabu Hatano 1 , Tatsuhiro Sakamoto 1 , Takuya Mochizuki 1 , Kazuaki Ishihara 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2019-04-01 , DOI: 10.1002/ajoc.201900104 Manabu Hatano 1 , Tatsuhiro Sakamoto 1 , Takuya Mochizuki 1 , Kazuaki Ishihara 1
Affiliation
|
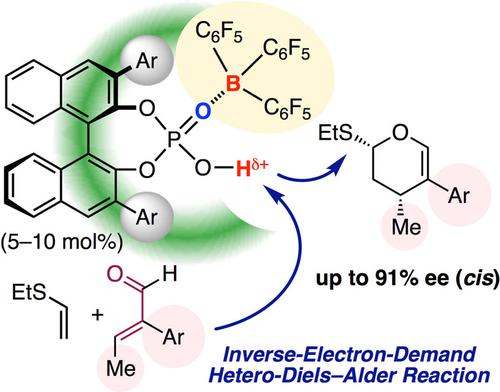
In the enantioselective inverse‐electron‐demand hetero‐Diels‐Alder reaction, simple α,β‐unsaturated aldehydes (i. e., acroleins) are still challenging substrates, unlike α,β‐unsaturated carbonyl compounds containing electron‐withdrawing groups. In the present study, the reaction of α‐aryl‐β‐alkyl‐substituted acroleins with ethyl vinyl sulfide was developed with the use of bulky chiral supramolecular Brønsted acid catalysts, such as tris(pentafluorophenyl)borane‐assisted chiral phosphoric acid catalysts. As a result, cis‐cycloadducts as optically active 3,4‐dihydro‐2H‐pyrans were exclusively obtained in high yields with high enantioselectivities via the favored endo orbital approach. An obtained optically active cis‐isomer could be transformed into the corresponding trans‐isomer without a loss of enantiopurity by O,S‐acetal epimerization. Moreover, transformations to synthetically useful optically active epoxide and δ‐valerolactone are also demonstrated.
中文翻译:

三(五氟苯基)硼烷辅助的手性磷酸催化剂,用于α,β取代的丙烯醛的对映选择性反电子-需求杂-Diels-Alder反应
在对映选择性逆电子需求杂Diels-Alder反应中,与含有吸电子基团的α,β-不饱和羰基化合物不同,简单的α,β-不饱和醛(即丙烯醛)仍是具有挑战性的底物。在本研究中,通过使用庞大的手性超分子布朗斯台德酸催化剂,例如三(五氟苯基)硼烷辅助的手性磷酸催化剂,开发了α-芳基-β-烷基取代的丙烯醛与乙基乙烯基硫化物的反应。结果,顺式环加合物作为旋光的3,4-二氢-2 H-吡喃通过有利的内轨道方法以高收率和高对映选择性独家获得。获得的光学活性顺式异构体可以转化为相应的反式异构体,而不会因O,S缩醛差向异构化而损失对映体纯度。此外,还证明了向合成有用的光学活性环氧化物和δ-戊内酯的转化。
更新日期:2019-04-01
中文翻译:

三(五氟苯基)硼烷辅助的手性磷酸催化剂,用于α,β取代的丙烯醛的对映选择性反电子-需求杂-Diels-Alder反应
在对映选择性逆电子需求杂Diels-Alder反应中,与含有吸电子基团的α,β-不饱和羰基化合物不同,简单的α,β-不饱和醛(即丙烯醛)仍是具有挑战性的底物。在本研究中,通过使用庞大的手性超分子布朗斯台德酸催化剂,例如三(五氟苯基)硼烷辅助的手性磷酸催化剂,开发了α-芳基-β-烷基取代的丙烯醛与乙基乙烯基硫化物的反应。结果,顺式环加合物作为旋光的3,4-二氢-2 H-吡喃通过有利的内轨道方法以高收率和高对映选择性独家获得。获得的光学活性顺式异构体可以转化为相应的反式异构体,而不会因O,S缩醛差向异构化而损失对映体纯度。此外,还证明了向合成有用的光学活性环氧化物和δ-戊内酯的转化。































 京公网安备 11010802027423号
京公网安备 11010802027423号