当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility of Sodium Oxalate in Concentrated Electrolyte Solutions
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-02-08 00:00:00 , DOI: 10.1021/acs.jced.7b00690 Glenn Hefter 1 , Andrew Tromans 2 , Peter M. May 3 , Erich Königsberger 3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-02-08 00:00:00 , DOI: 10.1021/acs.jced.7b00690 Glenn Hefter 1 , Andrew Tromans 2 , Peter M. May 3 , Erich Königsberger 3
Affiliation
|
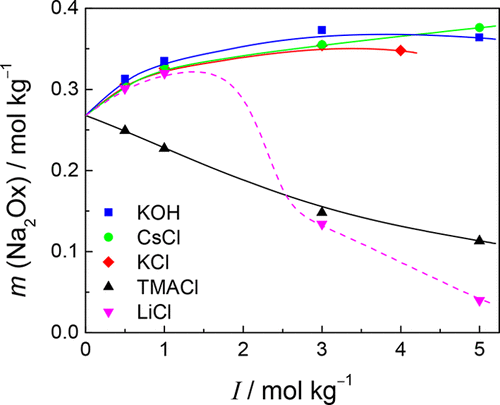
The solubility of solid sodium oxalate (Na2Ox) has been measured in a variety of concentrated aqueous electrolyte solutions at T = 298.15, 323.15, and 343.15 K by titration of dissolved oxalate with permanganate. The electrolyte solutions studied (not necessarily at all temperatures) were NaCl, NaClO4, NaOH, LiCl, KCl, Me4NCl, and KOH at concentrations ranging from approximately 0.5 mol·kg–1 to at least 5 mol·kg–1. Where comparisons were possible, the present results were in excellent agreement with literature data. The solubility of Na2Ox(s) decreased markedly with increasing concentrations of Na+(aq), due to the common ion effect. This decrease was almost independent of the electrolyte anion. A number of ternary mixtures of these electrolytes were also investigated at constant ionic strength. Consistent with the binary mixtures, the solubility of Na2Ox(s) showed almost no dependence on solution composition at constant Na+(aq) concentrations. Solubilities in non-Na+ media, with the exception of Me4NCl, showed small but regular increases with increasing concentration of added electrolyte, probably reflecting activity coefficient variations. The solubility data in certain Na+-containing media could be correlated accurately at all temperatures and concentrations using a relatively simple Pitzer model with interaction parameters for Na2Ox(aq) assumed to be identical to those available in the literature for Na2SO4(aq).
中文翻译:

草酸钠在浓电解液中的溶解度
通过用高锰酸盐滴定溶解的草酸盐,已在T = 298.15、323.15和343.15 K下,在各种浓电解质水溶液中测量了草酸钠(Na 2 Ox)固体的溶解度。所研究的电解质溶液(不一定在所有温度下)均为NaCl,NaClO 4,NaOH,LiCl,KCl,Me 4 NCl和KOH,浓度范围从大约0.5 mol·kg –1到至少5 mol·kg –1。在可能进行比较的情况下,目前的结果与文献数据非常吻合。随着Na +浓度的增加,Na 2 Ox(s)的溶解度显着降低(aq),由于常见的离子效应。这种降低几乎与电解质阴离子无关。还以恒定的离子强度研究了这些电解质的许多三元混合物。与二元混合物一致,在恒定的Na +(aq)浓度下,Na 2 Ox(s)的溶解度几乎不依赖于溶液组成。除Me 4 NCl外,在非Na +介质中的溶解度随添加电解质浓度的增加显示出少量但有规律的增加,这可能反映了活度系数的变化。在某些Na +中的溶解度数据可以使用相对简单的Pitzer模型在Na 2 Ox(aq)的相互作用参数与在文献中有关Na 2 SO 4(aq)的相互作用参数相同的情况下,在所有温度和浓度下精确地关联包含介质。
更新日期:2018-02-09
中文翻译:

草酸钠在浓电解液中的溶解度
通过用高锰酸盐滴定溶解的草酸盐,已在T = 298.15、323.15和343.15 K下,在各种浓电解质水溶液中测量了草酸钠(Na 2 Ox)固体的溶解度。所研究的电解质溶液(不一定在所有温度下)均为NaCl,NaClO 4,NaOH,LiCl,KCl,Me 4 NCl和KOH,浓度范围从大约0.5 mol·kg –1到至少5 mol·kg –1。在可能进行比较的情况下,目前的结果与文献数据非常吻合。随着Na +浓度的增加,Na 2 Ox(s)的溶解度显着降低(aq),由于常见的离子效应。这种降低几乎与电解质阴离子无关。还以恒定的离子强度研究了这些电解质的许多三元混合物。与二元混合物一致,在恒定的Na +(aq)浓度下,Na 2 Ox(s)的溶解度几乎不依赖于溶液组成。除Me 4 NCl外,在非Na +介质中的溶解度随添加电解质浓度的增加显示出少量但有规律的增加,这可能反映了活度系数的变化。在某些Na +中的溶解度数据可以使用相对简单的Pitzer模型在Na 2 Ox(aq)的相互作用参数与在文献中有关Na 2 SO 4(aq)的相互作用参数相同的情况下,在所有温度和浓度下精确地关联包含介质。















































 京公网安备 11010802027423号
京公网安备 11010802027423号