当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A catalytic asymmetric one-pot [3+2] cyclization/semipinacol rearrangement sequence: an efficient construction of a multi-substituted 3H-spiro[benzofuran-2,1′-cyclopentane] skeleton†
Chemical Communications ( IF 4.3 ) Pub Date : 2019-03-05 00:00:00 , DOI: 10.1039/c9cc00811j Lin Liu 1, 2, 3, 4 , Lin-Sheng Lei 1, 2, 3, 4 , Zong-Song Zhan 1, 2, 3, 4 , Si-Zhan Liu 1, 2, 3, 4 , Yu-Xiao Wang 1, 2, 3, 4 , Yong-Qiang Tu 1, 2, 3, 4 , Fu-Min Zhang 1, 2, 3, 4 , Xiao-Ming Zhang 1, 2, 3, 4 , Ai-Jun Ma 4, 5, 6, 7 , Shao-Hua Wang 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2019-03-05 00:00:00 , DOI: 10.1039/c9cc00811j Lin Liu 1, 2, 3, 4 , Lin-Sheng Lei 1, 2, 3, 4 , Zong-Song Zhan 1, 2, 3, 4 , Si-Zhan Liu 1, 2, 3, 4 , Yu-Xiao Wang 1, 2, 3, 4 , Yong-Qiang Tu 1, 2, 3, 4 , Fu-Min Zhang 1, 2, 3, 4 , Xiao-Ming Zhang 1, 2, 3, 4 , Ai-Jun Ma 4, 5, 6, 7 , Shao-Hua Wang 1, 2, 3, 4, 5
Affiliation
|
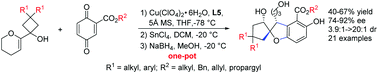
A facile and efficient method to form a chiral multi-substituted 3H-spiro[benzofuran-2,1′-cyclopentane] structural unit has been developed via a one-pot [3+2] cyclization/semipinacol rearrangement cascade. A catalysis system of Cu(II)/BOX has been used to efficiently construct a key stereogenic center via a cyclization between substituted benzoquinones and allylic alcohols affording the desired products in good yields and with excellent enantioselectivities and diastereoselectivities (21 examples; up to 67% yields; up to 92% ee and up to >20 : 1 dr). This method provides an alternative strategy for the synthesis of the corresponding bioactive molecules containing spiro[benzofurancyclopentane] skeleton units.
中文翻译:

催化不对称一锅[3 + 2]环化/ semipinacol重排序列:有效构建多取代的3 H-螺[苯并呋喃-2,1'-环戊烷]骨架†
通过一锅[3 + 2]环化/ Semipinacol重排级联开发了一种简便有效的方法,用于形成手性多取代的3 H-螺[苯并呋喃-2,1'-环戊烷]结构单元。Cu(II)/ BOX的催化系统已用于通过取代苯醌和烯丙醇之间的环化反应有效地构建关键的立体异构中心,从而以高收率提供所需产物,并具有出色的对映选择性和非对映选择性(21例;最高67%产量;最高ee为92%,最高> 20:1 dr)。该方法为合成包含螺[苯并呋喃环戊烷]骨架单元的相应生物活性分子提供了另一种策略。
更新日期:2019-03-05
中文翻译:

催化不对称一锅[3 + 2]环化/ semipinacol重排序列:有效构建多取代的3 H-螺[苯并呋喃-2,1'-环戊烷]骨架†
通过一锅[3 + 2]环化/ Semipinacol重排级联开发了一种简便有效的方法,用于形成手性多取代的3 H-螺[苯并呋喃-2,1'-环戊烷]结构单元。Cu(II)/ BOX的催化系统已用于通过取代苯醌和烯丙醇之间的环化反应有效地构建关键的立体异构中心,从而以高收率提供所需产物,并具有出色的对映选择性和非对映选择性(21例;最高67%产量;最高ee为92%,最高> 20:1 dr)。该方法为合成包含螺[苯并呋喃环戊烷]骨架单元的相应生物活性分子提供了另一种策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号