当前位置:
X-MOL 学术
›
Oncogenesis
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeted reduction of the EGFR protein, but not inhibition of its kinase activity, induces mitophagy and death of cancer cells through activation of mTORC2 and Akt.
Oncogenesis ( IF 5.9 ) Pub Date : 2018-01-23 , DOI: 10.1038/s41389-017-0021-7 Rajasekhara Reddy Katreddy 1 , Lakshmi Reddy Bollu 1 , Fei Su 1 , Na Xian 2 , Shivangi Srivastava 1 , Rintu Thomas 1 , Yubing Dai 1 , Bing Wu 2 , Yunlu Xu 2 , Michael A Rea 1 , James M Briggs 1 , Qingyuan Zhang 3 , Xiongbin Lu 4 , Gangxiong Huang 2, 5 , Zhang Weihua 1
Oncogenesis ( IF 5.9 ) Pub Date : 2018-01-23 , DOI: 10.1038/s41389-017-0021-7 Rajasekhara Reddy Katreddy 1 , Lakshmi Reddy Bollu 1 , Fei Su 1 , Na Xian 2 , Shivangi Srivastava 1 , Rintu Thomas 1 , Yubing Dai 1 , Bing Wu 2 , Yunlu Xu 2 , Michael A Rea 1 , James M Briggs 1 , Qingyuan Zhang 3 , Xiongbin Lu 4 , Gangxiong Huang 2, 5 , Zhang Weihua 1
Affiliation
|
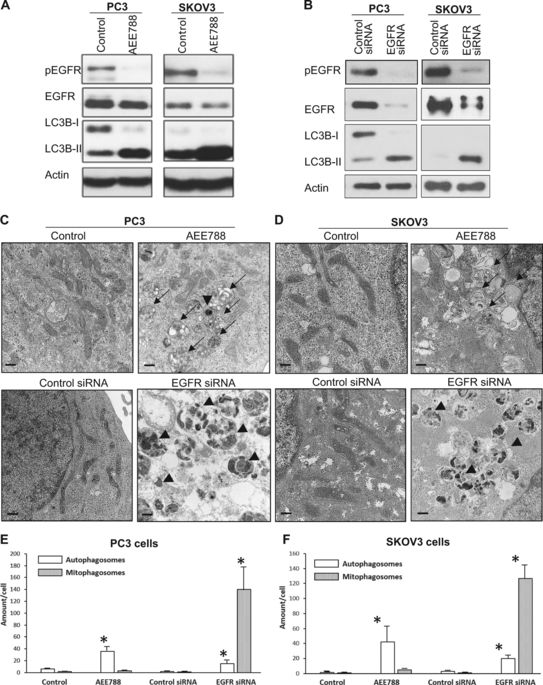
The oncogenic epidermal growth factor receptor (EGFR) is commonly overexpressed in solid cancers. The tyrosine kinase activity of EGFR has been a major therapeutic target for cancer; however, the efficacy of EGFR tyrosine kinase inhibitors to treat cancers has been challenged by innate and acquired resistance at the clinic. Accumulating evidence suggests that EGFR possesses kinase-independent pro-survival functions, and that cancer cells are more vulnerable to reduction of EGFR protein than to inhibition of its kinase activity. The molecular mechanism underlying loss-of-EGFR-induced cell death remains largely unknown. In this study, we show that, unlike inhibiting EGFR kinase activity that is known to induce pro-survival non-selective autophagy, downregulating EGFR protein, either by siRNA, or by a synthetic EGFR-downregulating peptide (Herdegradin), kills prostate and ovarian cancer cells via selective mitophagy by activating the mTORC2/Akt axis. Furthermore, Herdegradin induced mitophagy and inhibited the growth of orthotopic ovarian cancers in mice. This study identifies anti-mitophagy as a kinase-independent function of EGFR, reveals a novel function of mTORC2/Akt axis in promoting mitophagy in cancer cells, and offers a novel approach for pharmacological downregulation of EGFR protein as a potential treatment for EGFR-positive cancers.
中文翻译:

EGFR蛋白的靶向还原,但不抑制其激酶活性,通过激活mTORC2和Akt诱导线粒体吞噬和癌细胞死亡。
致癌表皮生长因子受体(EGFR)在实体癌中通常过表达。EGFR的酪氨酸激酶活性已成为癌症的主要治疗靶点。但是,EGFR酪氨酸激酶抑制剂治疗癌症的功效在临床上已受到先天和后天抵抗的挑战。越来越多的证据表明,EGFR具有不依赖激酶的促存活功能,并且癌细胞更容易受到EGFR蛋白还原的影响,而不是抑制其激酶活性。EGFR缺失引起的细胞死亡的分子机制仍然未知。在这项研究中,我们发现,与抑制EGFR激酶活性(已知可诱导生存前非选择性自噬)不同,siRNA可以下调EGFR蛋白,或通过合成的EGFR下调肽(Herdegradin),通过激活mTORC2 / Akt轴,通过选择性线粒吞噬杀死前列腺和卵巢癌细胞。此外,Herdegradin诱导线粒体吞噬并抑制小鼠原位卵巢癌的生长。这项研究确定抗丝裂蛋白是EGFR的激酶独立功能,揭示了mTORC2 / Akt轴在促进癌细胞线粒体中的新功能,并为EGFR蛋白的药理学下调提供了一种新的方法,将其作为EGFR阳性的潜在治疗方法癌症。
更新日期:2018-02-09
中文翻译:

EGFR蛋白的靶向还原,但不抑制其激酶活性,通过激活mTORC2和Akt诱导线粒体吞噬和癌细胞死亡。
致癌表皮生长因子受体(EGFR)在实体癌中通常过表达。EGFR的酪氨酸激酶活性已成为癌症的主要治疗靶点。但是,EGFR酪氨酸激酶抑制剂治疗癌症的功效在临床上已受到先天和后天抵抗的挑战。越来越多的证据表明,EGFR具有不依赖激酶的促存活功能,并且癌细胞更容易受到EGFR蛋白还原的影响,而不是抑制其激酶活性。EGFR缺失引起的细胞死亡的分子机制仍然未知。在这项研究中,我们发现,与抑制EGFR激酶活性(已知可诱导生存前非选择性自噬)不同,siRNA可以下调EGFR蛋白,或通过合成的EGFR下调肽(Herdegradin),通过激活mTORC2 / Akt轴,通过选择性线粒吞噬杀死前列腺和卵巢癌细胞。此外,Herdegradin诱导线粒体吞噬并抑制小鼠原位卵巢癌的生长。这项研究确定抗丝裂蛋白是EGFR的激酶独立功能,揭示了mTORC2 / Akt轴在促进癌细胞线粒体中的新功能,并为EGFR蛋白的药理学下调提供了一种新的方法,将其作为EGFR阳性的潜在治疗方法癌症。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号