当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparative Study on the Carbonation-Activated Calcium Silicates as Sustainable Binders: Reactivity, Mechanical Performance, and Microstructure
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-03-04 00:00:00 , DOI: 10.1021/acssuschemeng.8b06841 Yuandong Mu , Zhichao Liu , Fazhou Wang
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-03-04 00:00:00 , DOI: 10.1021/acssuschemeng.8b06841 Yuandong Mu , Zhichao Liu , Fazhou Wang
|
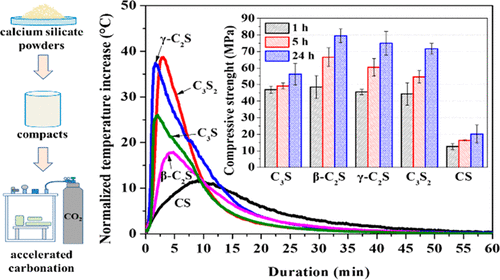
Calcium silicate minerals can react with CO2 to form calcium carbonate and have been proposed to be a sustainable binder as a potential CO2 sinker. In this study, the carbonation characteristics are comparatively assessed among calcium silicates having different calcium/silica (Ca/Si) ratios and polymorphs (CS, C3S2, γ-C2S, β-C2S, C3S). Calcium silicate compacts exposed to a 100% CO2 environment at a 0.4 MPa pressure were tested for carbonation temperature evolution, degree of carbonation (DOC), mechanical properties, and microstructural characterization. Results indicate γ-C2S is the most reactive, reaching a DOC of 50% in 24 h, followed by C3S2, CS, β-C2S, and C3S, which generally agrees with the pattern of the cumulative normalized temperature increase. Meanwhile, carbonated β-C2S compact attains the highest compressive strength of 80 MPa in 24 h, followed by γ-C2S, C3S2, and C3S, while CS only reaches 20 MPa. Calcite and aragonite are the preferable polymorphs of calcium carbonate in the carbonated C3S, γ-C2S, β-C2S, and C3S2, while only the carbonation of CS generates vaterite in addition to calcite and aragonite. The unreacted grains coated by a thin rim of calcium-modified silica gels are encapsulated by the continuous calcium carbonates, which composes the skeleton of the carbonated calcium silicates.
中文翻译:

碳酸活化硅酸钙作为可持续粘合剂的比较研究:反应性,机械性能和微观结构
硅酸钙矿物可以与CO 2反应形成碳酸钙,并且已被提议作为可持续的粘结剂作为潜在的CO 2沉降物。在这项研究中,碳酸化特性相对钙硅酸盐中评估具有不同钙/二氧化硅(钙/ Si)的比和多晶型物(CS,C 3小号2,γ-C 2 S,β-C 2 S,C 3 S) 。测试了在0.4 MPa压力下暴露于100%CO 2环境的硅酸钙压块的碳化温度演变,碳化程度(DOC),机械性能和微观结构表征。结果表明γ-C 2S是最具反应性的,达到了50%的在24小时DOC,接着为C 3小号2,CS,β-C 2 S,和C 3 S,其通常同意的累积归一化温度增加的图案。另一方面,碳酸β-C 2的紧凑型在无所获24小时80兆帕的最高压缩强度,接着γ-C 2 S,C 3小号2,和C 3 S,而只CS达到20兆帕。方解石和文石是在碳酸Ç碳酸钙的优选多晶型物3 S,γ-C 2 S,β-C 2 S,和C 3小号2,而除了方解石和文石之外,仅CS的碳化会生成球ate石。连续的碳酸钙包封了薄薄的钙改性硅胶边缘所覆盖的未反应颗粒,碳酸钙构成了碳酸硅酸钙的骨架。
更新日期:2019-03-04
中文翻译:

碳酸活化硅酸钙作为可持续粘合剂的比较研究:反应性,机械性能和微观结构
硅酸钙矿物可以与CO 2反应形成碳酸钙,并且已被提议作为可持续的粘结剂作为潜在的CO 2沉降物。在这项研究中,碳酸化特性相对钙硅酸盐中评估具有不同钙/二氧化硅(钙/ Si)的比和多晶型物(CS,C 3小号2,γ-C 2 S,β-C 2 S,C 3 S) 。测试了在0.4 MPa压力下暴露于100%CO 2环境的硅酸钙压块的碳化温度演变,碳化程度(DOC),机械性能和微观结构表征。结果表明γ-C 2S是最具反应性的,达到了50%的在24小时DOC,接着为C 3小号2,CS,β-C 2 S,和C 3 S,其通常同意的累积归一化温度增加的图案。另一方面,碳酸β-C 2的紧凑型在无所获24小时80兆帕的最高压缩强度,接着γ-C 2 S,C 3小号2,和C 3 S,而只CS达到20兆帕。方解石和文石是在碳酸Ç碳酸钙的优选多晶型物3 S,γ-C 2 S,β-C 2 S,和C 3小号2,而除了方解石和文石之外,仅CS的碳化会生成球ate石。连续的碳酸钙包封了薄薄的钙改性硅胶边缘所覆盖的未反应颗粒,碳酸钙构成了碳酸硅酸钙的骨架。

































 京公网安备 11010802027423号
京公网安备 11010802027423号