当前位置:
X-MOL 学术
›
Microchim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of poly(1,5-diaminonaphthalene) microparticles with abundant amino and imino groups as strong adsorbers for heavy metal ions
Microchimica Acta ( IF 5.3 ) Pub Date : 2019-03-02 , DOI: 10.1007/s00604-019-3330-z Xin-Gui Li , Mei-Rong Huang , Yuan-Bo Jiang , Jie Yu , Zikai He
Microchimica Acta ( IF 5.3 ) Pub Date : 2019-03-02 , DOI: 10.1007/s00604-019-3330-z Xin-Gui Li , Mei-Rong Huang , Yuan-Bo Jiang , Jie Yu , Zikai He
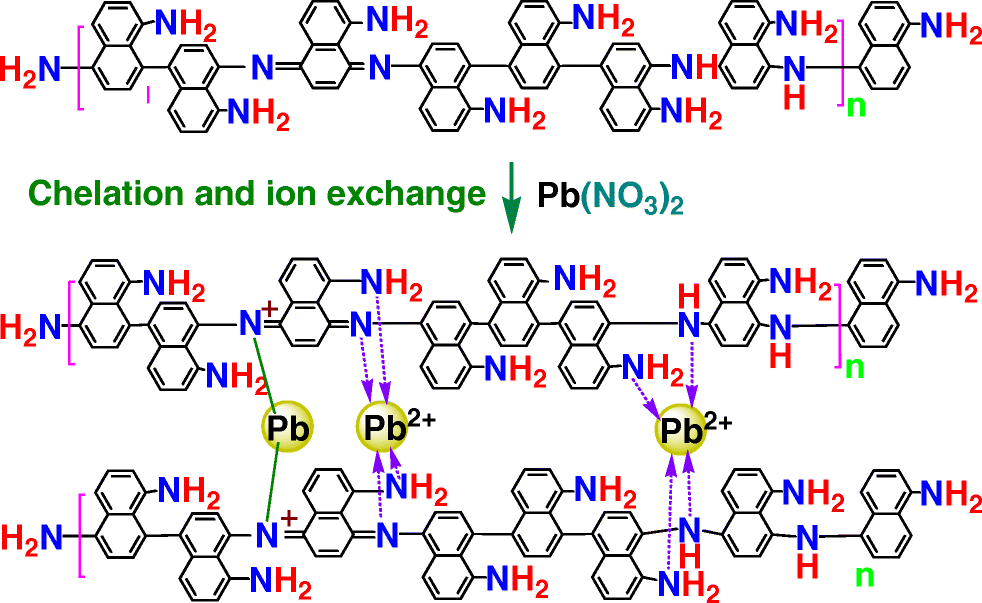
|
AbstractPoly(1,5-diaminonaphthalene) microparticles with abundant reactive amino and imino groups on their surface were synthesized by one-step oxidative polymerization of 1,5-diaminonaphthalene using ammonium persulfate as the oxidant. The molecular, supramolecular, and morphological structures of the microparticles were systematically characterized by IR and UV-vis spectroscopies, elementary analysis, wide-angle X-ray diffractometry, and transmission electron microscopy. The microparticles demonstrate electrical semiconductivity and high resistance to strong acid and alkali, and strong adsorption capability for lead(II), mercury(II), and silver(I) ions. The experimental conditions for adsorption of Pb(II) were optimized by varying the persulfate/monomer ratio, adsorption time, sorbent concentration, and pH value of the Pb(II) solution. The maximum adsorption capacity is 241 mg·g−1 for particles after a 24 h-exposure to a solution at an initial Pb(II) concentration of 29 mM. The adsorption data fit a Langmuir isotherm and follow a pseudo-second-order reaction kinetics. This indicates a chemical adsorption that is typical for a chelation interaction between Pb(II) and amino/imino groups on the sorbent. Graphical abstractPoly(1,5-diaminonaphthalene) microparticles with abundant functional amino and imino groups have been synthesized by one-step direct polymerization of non-volatile 1,5-diaminonaphthalene in aqueous medium for sustainable preparation of high-performance adsorbents to strongly adsorb lead(II), mercury(II), and silver(I) ions.
中文翻译:

具有丰富氨基和亚氨基的聚(1,5-二氨基萘)微粒作为重金属离子强吸附剂的合成
摘要 以过硫酸铵为氧化剂,通过一步氧化聚合1,5-二氨基萘,合成了表面具有丰富活性氨基和亚氨基的聚(1,5-二氨基萘)微粒。通过红外和紫外可见光谱、元素分析、广角 X 射线衍射和透射电子显微镜系统地表征了微粒的分子、超分子和形态结构。微粒表现出半导体导电性和对强酸和强碱的高耐受性,以及对铅(II)、汞(II)和银(I)离子的强吸附能力。通过改变过硫酸盐/单体比、吸附时间、吸附剂浓度和 Pb(II) 溶液的 pH 值来优化吸附 Pb(II) 的实验条件。在初始 Pb(II) 浓度为 29 mM 的溶液中暴露 24 小时后,颗粒的最大吸附容量为 241 mg·g-1。吸附数据符合朗缪尔等温线并遵循准二级反应动力学。这表明 Pb(II) 和吸附剂上的氨基/亚氨基之间的螯合相互作用是典型的化学吸附。通过在水性介质中一步直接聚合非挥发性 1,5-二氨基萘合成具有丰富功能氨基和亚氨基的聚(1,5-二氨基萘)微粒,可持续制备高性能吸附剂以强吸附铅(II)、汞 (II) 和银 (I) 离子。吸附数据符合朗缪尔等温线并遵循准二级反应动力学。这表明 Pb(II) 和吸附剂上的氨基/亚氨基之间的螯合相互作用是典型的化学吸附。通过在水性介质中一步直接聚合非挥发性 1,5-二氨基萘合成具有丰富功能氨基和亚氨基的聚(1,5-二氨基萘)微粒,可持续制备高性能吸附剂以强吸附铅(II)、汞 (II) 和银 (I) 离子。吸附数据符合朗缪尔等温线并遵循准二级反应动力学。这表明 Pb(II) 和吸附剂上的氨基/亚氨基之间的螯合相互作用是典型的化学吸附。通过在水性介质中一步直接聚合非挥发性 1,5-二氨基萘合成具有丰富功能氨基和亚氨基的聚(1,5-二氨基萘)微粒,可持续制备高性能吸附剂以强吸附铅(II)、汞 (II) 和银 (I) 离子。
更新日期:2019-03-02
中文翻译:

具有丰富氨基和亚氨基的聚(1,5-二氨基萘)微粒作为重金属离子强吸附剂的合成
摘要 以过硫酸铵为氧化剂,通过一步氧化聚合1,5-二氨基萘,合成了表面具有丰富活性氨基和亚氨基的聚(1,5-二氨基萘)微粒。通过红外和紫外可见光谱、元素分析、广角 X 射线衍射和透射电子显微镜系统地表征了微粒的分子、超分子和形态结构。微粒表现出半导体导电性和对强酸和强碱的高耐受性,以及对铅(II)、汞(II)和银(I)离子的强吸附能力。通过改变过硫酸盐/单体比、吸附时间、吸附剂浓度和 Pb(II) 溶液的 pH 值来优化吸附 Pb(II) 的实验条件。在初始 Pb(II) 浓度为 29 mM 的溶液中暴露 24 小时后,颗粒的最大吸附容量为 241 mg·g-1。吸附数据符合朗缪尔等温线并遵循准二级反应动力学。这表明 Pb(II) 和吸附剂上的氨基/亚氨基之间的螯合相互作用是典型的化学吸附。通过在水性介质中一步直接聚合非挥发性 1,5-二氨基萘合成具有丰富功能氨基和亚氨基的聚(1,5-二氨基萘)微粒,可持续制备高性能吸附剂以强吸附铅(II)、汞 (II) 和银 (I) 离子。吸附数据符合朗缪尔等温线并遵循准二级反应动力学。这表明 Pb(II) 和吸附剂上的氨基/亚氨基之间的螯合相互作用是典型的化学吸附。通过在水性介质中一步直接聚合非挥发性 1,5-二氨基萘合成具有丰富功能氨基和亚氨基的聚(1,5-二氨基萘)微粒,可持续制备高性能吸附剂以强吸附铅(II)、汞 (II) 和银 (I) 离子。吸附数据符合朗缪尔等温线并遵循准二级反应动力学。这表明 Pb(II) 和吸附剂上的氨基/亚氨基之间的螯合相互作用是典型的化学吸附。通过在水性介质中一步直接聚合非挥发性 1,5-二氨基萘合成具有丰富功能氨基和亚氨基的聚(1,5-二氨基萘)微粒,可持续制备高性能吸附剂以强吸附铅(II)、汞 (II) 和银 (I) 离子。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号