Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Knoevenagel/Tandem Knoevenagel and Michael Adducts of Cyclohexane-1,3-dione and Aryl Aldehydes: Synthesis, DFT Studies, Xanthine Oxidase Inhibitory Potential, and Molecular Modeling
ACS Omega ( IF 3.7 ) Pub Date : 2019-03-01 00:00:00 , DOI: 10.1021/acsomega.8b03060 Sahil Arora 1 , Gaurav Joshi 1 , Sourav Kalra 1 , Aabid Abdullah Wani 2 , Prasad V. Bharatam 2 , Pradeep Kumar 1 , Raj Kumar 1
ACS Omega ( IF 3.7 ) Pub Date : 2019-03-01 00:00:00 , DOI: 10.1021/acsomega.8b03060 Sahil Arora 1 , Gaurav Joshi 1 , Sourav Kalra 1 , Aabid Abdullah Wani 2 , Prasad V. Bharatam 2 , Pradeep Kumar 1 , Raj Kumar 1
Affiliation
|
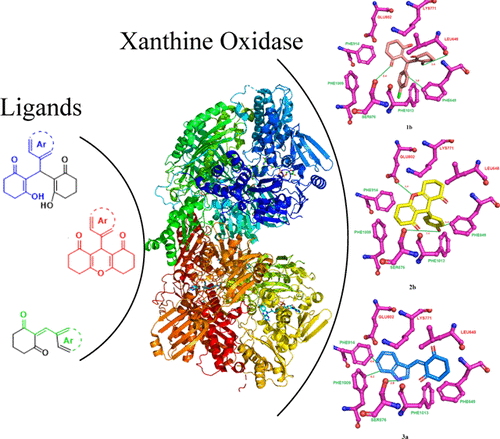
Xanthine oxidase (XO) plays a crucial role in the formation of uric acid by oxidative hydroxylation of purines. Herein, we report the design and synthesis of Knoevenagel/tandem Knoevenagel and Michael adducts of cyclohexane-1,3-dione and aryl aldehydes as nonpurine XO inhibitors derived from naturally occurring scaffolds. Density functional theory calculations highlighted the reaction pathways and reasoned the formation of tandem Knoevenagel and Michael adducts. The synthetics were assessed for their XO inhibitory potential, and among them, four compounds (1b, 1g, 2b, and 3a) were found to possess best IC50 values in the range of 3.66–4.98 μM. Interestingly, Knoevenagel adducts exhibited a competitive-type inhibition, whereas tandem Knoevenagel and Michael adducts produced a noncompetitive mode of inhibition. The compounds were capable of reducing the H2O2 levels induced by XO, both in normal and cancer cells with no significant cytotoxicity. Molecular modeling studies highlighted the role of interactions of compounds with residual amino acids of the XO active site and also corroborated with the observed structure–activity relationship.
中文翻译:

环己烷-1,3-二酮和芳醛的Knoevenagel /串联Knoevenagel和Michael加合物:合成,DFT研究,黄嘌呤氧化酶抑制潜能和分子建模
黄嘌呤氧化酶(XO)在嘌呤的氧化羟基化作用中形成尿酸起着至关重要的作用。在这里,我们报告设计和合成的Knoevenagel /串联Knoevenagel和环己烷-1,3-二酮和芳基醛的迈克尔加合物作为衍生自天然支架的非嘌呤XO抑制剂。密度泛函理论计算突出了反应途径,并推理了串联的Knoevenagel和Michael加合物的形成。对合成物的XO抑制潜力进行了评估,发现其中的四种化合物(1b,1g,2b和3a)具有最佳的IC 50值。值在3.66–4.98μM的范围内。有趣的是,Knoevenagel加合物具有竞争性抑制作用,而串联的Knoevenagel和Michael加合物则具有非竞争性抑制作用。该化合物在正常细胞和癌细胞中均能够降低XO诱导的H 2 O 2水平,而没有明显的细胞毒性。分子建模研究突出了化合物与XO活性位点残留氨基酸的相互作用的作用,并且与观察到的结构-活性关系得到了证实。
更新日期:2019-03-01
中文翻译:

环己烷-1,3-二酮和芳醛的Knoevenagel /串联Knoevenagel和Michael加合物:合成,DFT研究,黄嘌呤氧化酶抑制潜能和分子建模
黄嘌呤氧化酶(XO)在嘌呤的氧化羟基化作用中形成尿酸起着至关重要的作用。在这里,我们报告设计和合成的Knoevenagel /串联Knoevenagel和环己烷-1,3-二酮和芳基醛的迈克尔加合物作为衍生自天然支架的非嘌呤XO抑制剂。密度泛函理论计算突出了反应途径,并推理了串联的Knoevenagel和Michael加合物的形成。对合成物的XO抑制潜力进行了评估,发现其中的四种化合物(1b,1g,2b和3a)具有最佳的IC 50值。值在3.66–4.98μM的范围内。有趣的是,Knoevenagel加合物具有竞争性抑制作用,而串联的Knoevenagel和Michael加合物则具有非竞争性抑制作用。该化合物在正常细胞和癌细胞中均能够降低XO诱导的H 2 O 2水平,而没有明显的细胞毒性。分子建模研究突出了化合物与XO活性位点残留氨基酸的相互作用的作用,并且与观察到的结构-活性关系得到了证实。































 京公网安备 11010802027423号
京公网安备 11010802027423号