Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphorylation of Syntaxin 17 by TBK1 Controls Autophagy Initiation.
Developmental Cell ( IF 10.7 ) Pub Date : 2019-02-28 , DOI: 10.1016/j.devcel.2019.01.027 Suresh Kumar 1 , Yuexi Gu 1 , Yakubu Princely Abudu 2 , Jack-Ansgar Bruun 2 , Ashish Jain 3 , Farzin Farzam 4 , Michal Mudd 1 , Jan Haug Anonsen 5 , Tor Erik Rusten 3 , Gary Kasof 6 , Nicholas Ktistakis 7 , Keith A Lidke 4 , Terje Johansen 2 , Vojo Deretic 1
Developmental Cell ( IF 10.7 ) Pub Date : 2019-02-28 , DOI: 10.1016/j.devcel.2019.01.027 Suresh Kumar 1 , Yuexi Gu 1 , Yakubu Princely Abudu 2 , Jack-Ansgar Bruun 2 , Ashish Jain 3 , Farzin Farzam 4 , Michal Mudd 1 , Jan Haug Anonsen 5 , Tor Erik Rusten 3 , Gary Kasof 6 , Nicholas Ktistakis 7 , Keith A Lidke 4 , Terje Johansen 2 , Vojo Deretic 1
Affiliation
|
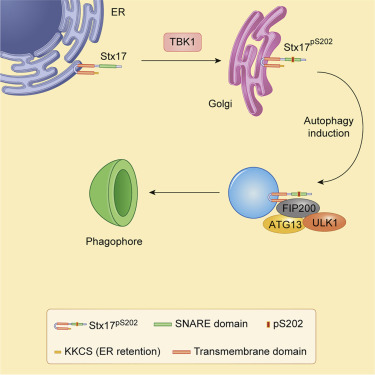
Syntaxin 17 (Stx17) has been implicated in autophagosome-lysosome fusion. Here, we report that Stx17 functions in assembly of protein complexes during autophagy initiation. Stx17 is phosphorylated by TBK1 whereby phospho-Stx17 controls the formation of the ATG13+FIP200+ mammalian pre-autophagosomal structure (mPAS) in response to induction of autophagy. TBK1 phosphorylates Stx17 at S202. During autophagy induction, Stx17pS202 transfers from the Golgi, where its steady-state pools localize, to the ATG13+FIP200+ mPAS. Stx17pS202 was in complexes with ATG13 and FIP200, whereas its non-phosphorylatable mutant Stx17S202A was not. Stx17 or TBK1 knockouts blocked ATG13 and FIP200 puncta formation. Stx17 or TBK1 knockouts reduced the formation of ATG13 protein complexes with FIP200 and ULK1. Endogenous Stx17pS202 colocalized with LC3B following induction of autophagy. Stx17 knockout diminished LC3 response and reduced sequestration of the prototypical bulk autophagy cargo lactate dehydrogenase. We conclude that Stx17 is a TBK1 substrate and that together they orchestrate assembly of mPAS.
中文翻译:

TBK1对Syntaxin 17的磷酸化作用可控制自噬的启动。
Syntaxin 17(Stx17)与自噬小体-溶酶体融合有关。在这里,我们报告说Stx17在自噬启动过程中蛋白质复合体组装中的功能。Stx17被TBK1磷酸化,从而磷酸化Stx17响应自噬的诱导而控制ATG13 + FIP200 +哺乳动物前自噬体结构(mPAS)的形成。在S202,TBK1使Stx17磷酸化。在自噬诱导过程中,Stx17pS202从其稳态池所在的高尔基体转移到ATG13 + FIP200 + mPAS。Stx17pS202与ATG13和FIP200形成复合物,而其不可磷酸化的突变体Stx17S202A没有。Stx17或TBK1敲除阻止了ATG13和FIP200点的形成。Stx17或TBK1敲除减少了FIP200和ULK1的ATG13蛋白复合物的形成。自噬诱导后内源性Stx17pS202与LC3B共定位。Stx17基因敲除减少了LC3反应,并减少了典型的散装自噬货物乳酸脱氢酶的螯合。我们得出的结论是,Stx17是TBK1底物,它们共同策划了mPAS的组装。
更新日期:2019-03-13
中文翻译:

TBK1对Syntaxin 17的磷酸化作用可控制自噬的启动。
Syntaxin 17(Stx17)与自噬小体-溶酶体融合有关。在这里,我们报告说Stx17在自噬启动过程中蛋白质复合体组装中的功能。Stx17被TBK1磷酸化,从而磷酸化Stx17响应自噬的诱导而控制ATG13 + FIP200 +哺乳动物前自噬体结构(mPAS)的形成。在S202,TBK1使Stx17磷酸化。在自噬诱导过程中,Stx17pS202从其稳态池所在的高尔基体转移到ATG13 + FIP200 + mPAS。Stx17pS202与ATG13和FIP200形成复合物,而其不可磷酸化的突变体Stx17S202A没有。Stx17或TBK1敲除阻止了ATG13和FIP200点的形成。Stx17或TBK1敲除减少了FIP200和ULK1的ATG13蛋白复合物的形成。自噬诱导后内源性Stx17pS202与LC3B共定位。Stx17基因敲除减少了LC3反应,并减少了典型的散装自噬货物乳酸脱氢酶的螯合。我们得出的结论是,Stx17是TBK1底物,它们共同策划了mPAS的组装。































 京公网安备 11010802027423号
京公网安备 11010802027423号